Complications related to endoscopic lung volume reduction for emphysema with endobronchial valves: results of a multicenter study
- PMID: 30450237
- PMCID: PMC6204336
- DOI: 10.21037/jtd.2018.06.69
Complications related to endoscopic lung volume reduction for emphysema with endobronchial valves: results of a multicenter study
Abstract
Background: Despite bronchoscopic lung volume reduction (BLVR) with valves is a minimally invasive treatment for emphysema, it can associate with some complications. We aimed at evaluating the rate and type of complications related to valve treatment and their impact on clinical outcomes.
Methods: It is a retrospective multicenter study including all consecutive patients with severe heterogeneous emphysema undergoing BLVR with endobronchial valve treatment and developed any complications related to this procedure. The type of complication, the time of onset, the treatment required and the out-come were evaluated. Response to treatment was assessed according to the minimal clinically important difference (MCID) as follows: an improvement of ≥15% in forced expiratory volume in one second (FEV1); of -8% in residual volume (RV); of ≥26 m in 6-minnute walking distance (6MWD); and of ≥4 points on the St. George's Respiratory Questionnaire (SGRQ). Target lobe volume reduction (TLVR) ≥350 mL was considered significant.
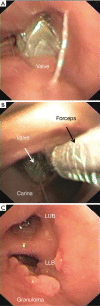
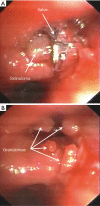
Results: One hundred and seven out of 423 (25.3%) treated patients had complications related to valve treatment including pneumothorax (17.3%); pneumonia (1.7%), chronic obstructive pulmonary disease (COPD) exacerbation (0.9%), respiratory failure (1.4%), valve migration (2.1%), and hemoptysis (1.9%). In all cases complications resolved with appropriate treatment including removal of valves in 21/107 cases (19.6%). Patients with TLVR ≥350 mL (n=64) vs. those <350 mL (n=43) had a statistically significant higher improvement in FEV1 (19.0%±3.9% vs. 3.0%±0.9%; P=0.0003); in RV (-10.0%±4.8% vs. -4.0%±2.9%; P=0.002); in 6MWD (33.0±19.0 vs. 12.0±6.3 metres; P=0.001); and in SGRQ (-15.0±2.9 vs. -8.0±3.5 points; P=0.01). Only patients with TLVR ≥350 mL met or exceeded the MCID cut-off criteria for FEV1 (19.0%±3.9%), RV (-10.0%±4.8%), 6MWT (33.0±19.0 metres), and SGQR (-15.0±2.9 points). Five patients (1.2%) died during follow-up for causes not related to valves treatment neither to any of the complications described.
Conclusions: Valve treatment is a safe and reversible procedure. The presence of complications seems not to have a significant impact on clinical outcome in patients with lobar atelectasis. Due to poor clinical conditions and possible complications, BLVR should be performed in high volume centers with a multidisciplinary approach.
Keywords: Zephyr endo-bronchial valves; bronchoscopic lung volume reduction (BLVR); emphysema.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous
