Structure of an engineered intein reveals thiazoline ring and provides mechanistic insight
- PMID: 30450538
- PMCID: PMC6399034
- DOI: 10.1002/bit.26875
Structure of an engineered intein reveals thiazoline ring and provides mechanistic insight
Abstract
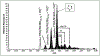
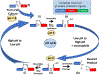
We have engineered an intein which spontaneously and reversibly forms a thiazoline ring at the native N-terminal Lys-Cys splice junction. We identified conditions to stablize the thiazoline ring and provided the first crystallographic evidence, at 1.54 Å resolution, for its existence at an intein active site. The finding bolsters evidence for a tetrahedral oxythiazolidine splicing intermediate. In addition, the pivotal mutation maps to a highly conserved B-block threonine, which is now seen to play a causative role not only in ground-state destabilization of the scissile N-terminal peptide bond, but also in steering the tetrahedral intermediate toward thioester formation, giving new insight into the splicing mechanism. We demonstrated the stability of the thiazoline ring at neutral pH as well as sensitivity to hydrolytic ring opening under acidic conditions. A pH cycling strategy to control N-terminal cleavage is proposed, which may be of interest for biotechnological applications requiring a splicing activity switch, such as for protein recovery in bioprocessing.
Keywords: cleavage control; molecular switch; protein splicing; thiazoline crystal structure.
© 2018 Wiley Periodicals, Inc.
Figures






References
-
- Bloudoff K, Schmeing TM. 2017. Structural and functional aspects of the nonribosomal peptide synthetase condensation domain superfamily: discovery, dissection and diversity. Biochim Biophys Acta, 1865, 1587–1604. - PubMed
-
- Bocker JK, Friedel K, Matern JC, Bachmann AL, Mootz HD. 2015. Generation of a genetically encoded, photoactivatable intein for the controlled production of cyclic peptides. Angew Chem Int Ed Engl, 54, 2116–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

