Evolutionary significance of the microbial assemblages of large benthic Foraminifera
- PMID: 30450723
- PMCID: PMC7379505
- DOI: 10.1111/brv.12482
Evolutionary significance of the microbial assemblages of large benthic Foraminifera
Abstract
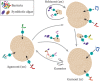
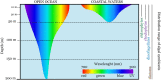
Large benthic Foraminifera (LBF) are major carbonate producers on coral reefs, and are hosts to a diverse symbiotic microbial community. During warm episodes in the geological past, these reef-building organisms expanded their geographical ranges as subtropical and tropical belts moved into higher latitudes. During these range-expansion periods, LBF were the most prolific carbonate producers on reefs, dominating shallow carbonate platforms over reef-building corals. Even though the fossil and modern distributions of groups of species that harbour different types of symbionts are known, the nature, mechanisms, and factors that influence their occurrence remain elusive. Furthermore, the presence of a diverse and persistent bacterial community has only recently gained attention. We examined recent advances in molecular identification of prokaryotic (i.e. bacteria) and eukaryotic (i.e. microalgae) associates, and palaeoecology, and place the partnership with bacteria and algae in the context of climate change. In critically reviewing the available fossil and modern data on symbiosis, we reveal a crucial role of microalgae in the response of LBF to ocean warming, and their capacity to colonise a variety of habitats, across both latitudes and broad depth ranges. Symbiont identity is a key factor enabling LBF to expand their geographic ranges when the sea-surface temperature increases. Our analyses showed that over the past 66 million years (My), diatom-bearing species were dominant in reef environments. The modern record shows that these species display a stable, persistent eukaryotic assemblage across their geographic distribution range, and are less dependent on symbiotic photosynthesis for survival. By contrast, dinoflagellate and chlorophytic species, which show a provincial distribution, tend to have a more flexible eukaryotic community throughout their range. This group is more dependent on their symbionts, and flexibility in their symbiosis is likely to be the driving force behind their evolutionary history, as they form a monophyletic group originating from a rhodophyte-bearing ancestor. The study of bacterial assemblages, while still in its infancy, is a promising field of study. Bacterial communities are likely to be shaped by the local environment, although a core bacterial microbiome is found in species with global distributions. Cryptic speciation is also an important factor that must be taken into consideration. As global warming intensifies, genetic divergence in hosts in addition to the range of flexibility/specificity within host-symbiont associations will be important elements in the continued evolutionary success of LBF species in a wide range of environments. Based on fossil and modern data, we conclude that the microbiome, which includes both algal and bacterial partners, is a key factor influencing the evolution of LBF. As a result, the microbiome assists LBF in colonising a wide range of habitats, and allowed them to become the most important calcifiers on shallow platforms worldwide during periods of ocean warming in the geologic past. Since LBF are crucial ecosystem engineers and prolific carbonate producers, the microbiome is a critical component that will play a central role in the responses of LBF to a changing ocean, and ultimately in shaping the future of coral reefs.
Keywords: Cenozoic; climate change; coral reefs; microbiome; ocean warming; symbiosis.
© 2018 The Authors. Biological Reviews published by John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society.
Figures




Similar articles
-
Fate of calcifying tropical symbiont-bearing large benthic foraminifera: living sands in a changing ocean.Biol Bull. 2014 Jun;226(3):169-86. doi: 10.1086/BBLv226n3p169. Biol Bull. 2014. PMID: 25070863 Review.
-
Symbiosis and microbiome flexibility in calcifying benthic foraminifera of the Great Barrier Reef.Microbiome. 2017 Mar 23;5(1):38. doi: 10.1186/s40168-017-0257-7. Microbiome. 2017. PMID: 28335814 Free PMC article.
-
Diversity and flexibility of algal symbiont community in globally distributed larger benthic foraminifera of the genus Amphistegina.BMC Microbiol. 2021 Sep 6;21(1):243. doi: 10.1186/s12866-021-02299-8. BMC Microbiol. 2021. PMID: 34488648 Free PMC article.
-
Bleaching-Associated Changes in the Microbiome of Large Benthic Foraminifera of the Great Barrier Reef, Australia.Front Microbiol. 2018 Oct 9;9:2404. doi: 10.3389/fmicb.2018.02404. eCollection 2018. Front Microbiol. 2018. PMID: 30356788 Free PMC article.
-
Global diversity patterns of larger benthic foraminifera under future climate change.Glob Chang Biol. 2023 Feb;29(4):969-981. doi: 10.1111/gcb.16535. Epub 2022 Nov 29. Glob Chang Biol. 2023. PMID: 36413112 Review.
Cited by
-
Shallow water foraminifera from Niue and Beveridge Reef (South Pacific): insights into ecological significance and ecosystem integrity.R Soc Open Sci. 2024 Jan 10;11(1):230997. doi: 10.1098/rsos.230997. eCollection 2024 Jan. R Soc Open Sci. 2024. PMID: 38204782 Free PMC article.
-
Lost in translation: conserved amino acid usage despite extreme codon bias in foraminifera.mBio. 2025 Apr 9;16(4):e0391624. doi: 10.1128/mbio.03916-24. Epub 2025 Mar 5. mBio. 2025. PMID: 40042280 Free PMC article.
-
Unravelling Evolutionary and Ecological Insights of Foraminifera by Using Next Generation Sequencing: A Review.Biochem Genet. 2025 Jul 15. doi: 10.1007/s10528-025-11200-5. Online ahead of print. Biochem Genet. 2025. PMID: 40665121 Review.
-
The change in metabolic activity of a large benthic foraminifera as a function of light supply.Sci Rep. 2023 May 22;13(1):8240. doi: 10.1038/s41598-023-35342-x. Sci Rep. 2023. PMID: 37217641 Free PMC article.
-
Integrating morphology and metagenomics to understand taxonomic variability of Amphisorus (Foraminifera, Miliolida) from Western Australia and Indonesia.PLoS One. 2021 Jan 4;16(1):e0244616. doi: 10.1371/journal.pone.0244616. eCollection 2021. PLoS One. 2021. PMID: 33395419 Free PMC article.
References
-
- Adams, C. G. , Lee, D. E. & Rosen, B. R. (1990). Conflicting isotopic and biotic evidence for tropical sea‐surface temperatures during the Tertiary. Palaeogeography Palaeoclimatology Palaeoecology 77, 289–313.
-
- Ainsworth, T. & Gates, R. D. (2016). Corals' microbial sentinels. Science 352, 1518–1519. - PubMed
-
- Ainsworth, T. D. , Krause, L. , Bridge, T. , Torda, G. , Raina, J. B. , Zakrzewski, M. , Gates, R. D. , Padilla‐Gamino, J. L. , Spalding, H. L. , Smith, C. , Woolsey, E. S. , Bourne, D. G. , Bongaerts, P. , Hoegh‐Guldberg, O. & Leggat, W. (2015). The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. ISME Journal 9, 2261–2274. - PMC - PubMed
-
- Ainsworth, T. D. , Thurber, R. V. & Gates, R. D. (2010). The future of coral reefs: a microbial perspective. Trends in Ecology & Evolution 25, 233–240. - PubMed
-
- Al‐Wosabi, M. A. , Mohammed, M. A. & Basardah, F. (2017). Taxonomy and distribution of recent benthic foraminifera from Bir Ali Beach, Shabwah Governorate, Arabian Sea, Yemen. Geological Bulletin of Turkey 60, 383–432.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

