Long-term low salt diet increases blood pressure by activation of the renin-angiotensin and sympathetic nervous systems
- PMID: 30451012
- PMCID: PMC6525650
- DOI: 10.1080/10641963.2018.1545850
Long-term low salt diet increases blood pressure by activation of the renin-angiotensin and sympathetic nervous systems
Abstract
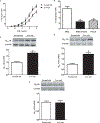
Background The aim of this study was to investigate the effect of long-term low salt diet on blood pressure and its underlying mechanisms.Methods Male Sprague-Dawley (SD) rats were divided into normal salt diet group (0.4%) and low salt diet group (0.04%). Blood pressure was measured with the non-invasive tail-cuff method. The contractile response of isolated mesenteric arteries was measured using a small vessel myograph. The effects on renal function of the intrarenal arterial infusion of candesartan (10 μg/kg/min), an angiotensin II receptor type 1 (AT1R) antagonist, were also measured. The expressions of renal AT1R and mesenteric arterial α1A, α1B, and α1D adrenergic receptors were quantified by immunoblotting. Plasma levels of angiotensin II were also measured.Results Systolic blood pressure was significantly increased after 8 weeks of low salt diet. There were no obvious differences in the renal structure between the low and normal salt diet groups. However, the plasma angiotensin II levels and renal AT1R expression were higher in low than normal salt diet group. The intrarenal arterial infusion of candesartan increased urine flow and sodium excretion to a greater extent in the low than normal salt diet group. The expressions of α1A and α1D, but not α1B, adrenergic receptors, and phenylephrine-induced contraction were increased in mesenteric arteries from the low salt, relative to the normal salt diet group.Conclusion Activation of the renin-angiotensin and sympathetic nervous systems may be involved in the pathogenesis of long-term low salt diet-induced hypertension.
Keywords: Hypertension; low salt diet; renin-angiotensin system; sodium excretion; sympathetic nervous system; vascular contraction.
Conflict of interest statement
Disclosure statement
The authors declare that they have no competing interests. This manuscript is an original contribution not previously published, and not under consideration for publication elsewhere.
Figures
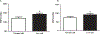


References
-
- Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC Jr, Vafiadis DK, Van Horn LV. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation 2011;123:1138–43. 10.1161/CIR.0b013e31820d0793. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
