Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities
- PMID: 30451076
- PMCID: PMC6391637
- DOI: 10.2217/nnm-2018-0120
Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities
Abstract
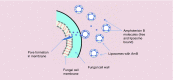
There has been a revolution in nanotechnology and nanomedicine. Since 1980, there has been a remarkable increase in approved nano-based pharmaceutical products. These novel nano-based systems can either be therapeutic agents themselves, or else act as vehicles to carry different active pharmaceutical agents into specific parts of the body. Currently marketed nanostructures include nanocrystals, liposomes and lipid nanoparticles, PEGylated polymeric nanodrugs, other polymers, protein-based nanoparticles and metal-based nanoparticles. A range of issues must be addressed in the development of these nanostructures. Ethics, market size, possibility of market failure, costs and commercial development, are some topics which are on the table to be discussed. After passing all the ethical and biological assessments, and satisfying the investors as to future profitability, only a handful of these nanoformulations, successfully obtained marketing approval. We survey the range of nanomedicines that have received regulatory approval and are marketed. We discuss ethics, costs, commercial development and possible market failure. We estimate the global nanomedicine market size and future growth. Our goal is to summarize the different approved nanoformulations on the market, and briefly cover the challenges and future outlook.
Keywords: nanopharmaceuticals; drug delivery; market size; nanocrystal; nanodrug; nanoliposome; nanomedicine; nanopolymer; nanotechnology; regulatory approval.
Conflict of interest statement
MR Hamblin was supported by US NIH grants R01AI050875 and R21AI121700. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures






References
-
- Eric DK. Engines of Creation. The Coming Era of Nanotechnology. Doubleday; NY, USA: 1986.
-
- Feynman RP. There's plenty of room at the bottom. Eng. Sci. 1960;23(5):22–36.
-
•• The very first inkling of what would become the nanotechology revolution.
-
- Devreese JT. Importance of nanosensors: Feynman's vision and the birth of nanotechnology. MRS Bull. 2007;32(09):718–725.
-
- Qu X, Alvarez PJ, Li Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013;47(12):3931–3946. - PubMed
-
- Sawhney A, Condon B, Singh K, Pang S, Li G, Hui D. Modern applications of nanotechnology in textiles. Textile Res. J. 2008;78(8):731–739.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
