Periocular topotecan for vitreous seeds in retinoblastoma
- PMID: 30451190
- PMCID: PMC6256916
- DOI: 10.4103/ijo.IJO_737_18
Periocular topotecan for vitreous seeds in retinoblastoma
Abstract
Purpose: Refractory or recurrent vitreous seeds account for a large proportion of failure of eye salvage in retinoblastoma. The purpose of this study is to evaluate the efficacy of periocular topotecan (POT) in the management of vitreous seeds in retinoblastoma.
Methods: Retrospective, interventional study of patients with retinoblastoma with vitreous seeds who received POT concurrent with intravenous chemotherapy (IVC).
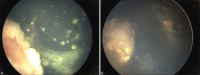
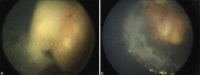
Results: Thirty-eight eyes of 35 patients received POT. Five eyes (13%) belonged to International Classification of Retinoblastoma group C, 23 eyes (61%) belonged to group D, and 10 eyes (26%) belonged to group E. Primary treatment included IVC with a combination of carboplatin, etoposide, and vincristine for a mean of 6 cycles (median 6; range 6-9). Concurrent to IVC from the fourth cycle onward, all patients received POT. Focal vitreous seeds were present in 20 eyes (53%) which received a mean of 3 injections (median 3; range 1-7). Diffuse vitreous seeds were present in 18 eyes (47%) which received a mean of 4 injections (median 5; range 1-7). At a mean follow-up of 8.5 months (median 5 months; range 1-15 months), regression of focal and diffuse vitreous seeds was achieved in 16 eyes (80%) and 8 eyes (44%), respectively. In all, 24 eyes (63%) had complete remission of vitreous seeds with POT given concurrently with IVC. Eye salvage was possible in 19 eyes (95%) with focal vitreous seeds and 12 eyes (68%) with diffuse VS. Enucleation was necessary for persistent vitreous seeds and viable tumor in five eyes (13%), viable tumor alone in one eye (0.02%), and recurrent vitreous seeds in one eye (0.02%). None of the patients developed systemic metastasis.
Conclusion: POT administered concurrent with IVC is safe and effective in the initial management of vitreous seeds.
Keywords: Periocular injection; retinoblastoma; topotecan; vitreous seeds.
Conflict of interest statement
There are no conflicts of interest
Figures

Comment in
-
Commentary: Periocular topotecan for retinoblastoma.Indian J Ophthalmol. 2018 Dec;66(12):1838-1839. doi: 10.4103/ijo.IJO_1626_18. Indian J Ophthalmol. 2018. PMID: 30451191 Free PMC article. No abstract available.
References
-
- Shields CL, Douglass AM, Beggache M, Say EAT, Shields JA. Intravenous chemotherapy for active vitreous seeding from retinoblastoma: Outcomes after 192 consecutive injections. The 2015 Howard Naquin Lecture. Retina. 2016;36:1184–90. - PubMed
-
- Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–80. - PubMed
-
- Munier FL, Gaillard MC, Balmer A, Soliman S, Podilsky G, Moulin AP, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: From prohibition to conditional indications. Br J Ophthalmol. 2012;96:1078–83. - PubMed
-
- Shields CL, Honavar SG, Shields JA, Demirci H, Meadows AT, Naduvilath TJ. Factors predictive of recurrence of retinal tumors, vitreous seeds, and subretinal seeds following chemoreduction for retinoblastoma. Arch Ophthalmol. 2002;120:460–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

