Glycopyrronium tosylate in pediatric primary axillary hyperhidrosis: Post hoc analysis of efficacy and safety findings by age from two phase three randomized controlled trials
- PMID: 30451318
- PMCID: PMC6587744
- DOI: 10.1111/pde.13723
Glycopyrronium tosylate in pediatric primary axillary hyperhidrosis: Post hoc analysis of efficacy and safety findings by age from two phase three randomized controlled trials
Erratum in
-
Glycopyrronium tosylate in pediatric primary axillary hyperhidrosis: Post hoc analysis of efficacy and safety findings by age from two phase three randomized controlled trials.Pediatr Dermatol. 2019 May;36(3):424. doi: 10.1111/pde.13877. Pediatr Dermatol. 2019. PMID: 31099927 Free PMC article. No abstract available.
Abstract
Objectives: Hyperhidrosis in pediatric patients has been understudied. Post hoc analyses of two phase 3 randomized, vehicle-controlled, 4-week trials (ATMOS-1 [NCT02530281] and ATMOS-2 [NCT02530294]) were performed to assess efficacy and safety of topical anticholinergic glycopyrronium tosylate (GT) in pediatric patients.
Methods: Patients had primary axillary hyperhidrosis ≥ 6 months, average Axillary Sweating Daily Diary (ASDD/ASDD-Children [ASDD-C]) Item 2 (sweating severity) score ≥ 4, sweat production ≥ 50 mg/5 min (each axilla), and Hyperhidrosis Disease Severity Scale (HDSS) ≥ 3. Coprimary end points were ≥ 4-point improvement on ASDD/ASDD-C Item 2 (a validated patient-reported outcome) and change in gravimetrically measured sweat production at Week 4. Efficacy and safety data are shown through Week 4 for the pediatric (≥ 9 to ≤ 16 years) vs older (> 16 years) subgroups.
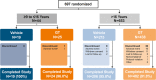
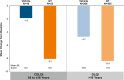
Results: Six hundred and ninety-seven patients were randomized in ATMOS-1/ATMOS-2 (GT, N = 463; vehicle, N = 234); 44 were ≥ 9 to ≤ 16 years (GT, n = 25; vehicle, n = 19). Baseline disease characteristics were generally similar across subgroups. GT-treated pediatric vs older patients had comparable improvements in ASDD/ASDD-C Item 2 (sweating severity) responder rate, HDSS responder rate (≥ 2-grade improvement]), sweat production, and quality of life (mean change from Baseline in Dermatology Life Quality Index [DLQI]/children's DLQI), with greater improvement vs vehicle. Treatment-emergent adverse events were similar between subgroups, and most were mild, transient, and infrequently led to discontinuation.
Conclusions: Topical, once-daily GT improved disease severity (ASDD/ASDD-C, HDSS), sweat production, and quality of life (DLQI), with similar findings in children, adults, and the pooled population. GT was well tolerated, and treatment-emergent adverse events were qualitatively similar between subgroups and consistent with other anticholinergics.
© 2018 The Authors. Pediatric Dermatology Published by Wiley Periodicals, Inc.
Conflict of interest statement
Dr. Hebert is a consultant for Dermira, Inc., and an employee of the UTHealth McGovern Medical School, Houston, which received compensation from Dermira, Inc., for study participation. Dr. Glaser is a consultant for Dermira, Inc., and an investigator for Allergan; Atacama Therapeutics; Brickell Biotech, Inc.; Galderma; and Revance Therapeutics, Inc. She has received honoraria for consulting with Allergan and Dermira, Inc. Dr. Green is an investigator for Brickell Biotech, Inc., and an advisory board member and investigator for Dermira, Inc. Dr. Werschler is a consultant and investigator for Dermira, Inc. Dr. Forsha is an investigator for Jordan Valley Dermatology and Research Center. Ms. Drew and Dr. Gopalan are employees of Dermira, Inc. Dr. Pariser received honoraria for consulting for Atacama Therapeutics; Brickell Biotech, Inc.; Biofrontera AG; Celgene Corporation; Dermira, Inc.; DUSA Pharmaceuticals, Inc.; LEO Pharma, Inc.; Novartis Pharmaceuticals Corporation; Promius Pharma; LLC; Regeneron Pharmaceuticals, Inc.; Sanofi; TDM SurgiTech, Inc.; TheraVida, Inc.; and Valeant Pharmaceuticals International, Inc. He received honoraria for advisory board participation for Pfizer, Inc. He received grants/research funding for serving as an investigator for Abbott Laboratories; Amgen, Inc.; Asana BioSciences; LLC; Brickell Biotech Inc.; Celgene Corporation; Dermavant Sciences, Inc.; Eli Lilly and Company; LEO Pharma, Inc.; Merck & Company, Inc.; Novartis Pharmaceuticals Corporation; Novo Nordisk A/S; Ortho Dermatologics, Inc.; Peplin, Inc.; Photocure ASA; Promius Pharma; LLC; Regeneron Pharmaceuticals, Inc.; Stiefel Laboratories; and Valeant Pharmaceuticals International, Inc. He received honoraria for serving as an investigator for LEO Pharma, Inc., and Pfizer, Inc.
Figures

a
ET forATMOS ‐1 andATMOS ‐2.bGravimetrically measured.
aPatient had five drug‐related events that led to discontinuation: mild vision blurred (bilateral), severe mydriasis (bilateral), severe dry mouth, severe urinary retention, and severe anhidrosis.
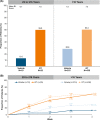
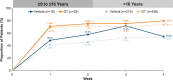
aGravimetrically measured average from the left and right axillae.

References
-
- Hebert A, Glaser DA, Ballard A, Pieretti L, de Trindade Almeida A, Pariser D. Prevalence of primary focal hyperhidrosis (PFHh) among teens 12‐17 in U.S. Population. Oral (Late‐Breaker) presented at 75th Annual Meeting of the American Academy of Dermatology; 2017; Orlando, FL.
-
- Amir M, Arish A, Weinstein Y, Pfeffer M, Levy Y. Impairment in quality of life among patients seeking surgery for hyperhidrosis (excessive sweating): preliminary results. Isr J Psychiatry Relat Sci. 2000;37(1):25‐31. - PubMed
-
- Cina CS, Clase CM. The Illness Intrusiveness Rating Scale: a measure of severity in individuals with hyperhidrosis. Qual Life Res. 1999;8(8):693‐698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

