Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials
- PMID: 30451345
- PMCID: PMC6526078
- DOI: 10.1002/jmri.26518
Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials
Abstract
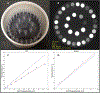
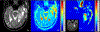
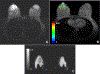
Physiological properties of tumors can be measured both in vivo and noninvasively by diffusion-weighted imaging and dynamic contrast-enhanced magnetic resonance imaging. Although these techniques have been used for more than two decades to study tumor diffusion, perfusion, and/or permeability, the methods and studies on how to reduce measurement error and bias in the derived imaging metrics is still lacking in the literature. This is of paramount importance because the objective is to translate these quantitative imaging biomarkers (QIBs) into clinical trials, and ultimately in clinical practice. Standardization of the image acquisition using appropriate phantoms is the first step from a technical performance standpoint. The next step is to assess whether the imaging metrics have clinical value and meet the requirements for being a QIB as defined by the Radiological Society of North America's Quantitative Imaging Biomarkers Alliance (QIBA). The goal and mission of QIBA and the National Cancer Institute Quantitative Imaging Network (QIN) initiatives are to provide technical performance standards (QIBA profiles) and QIN tools for producing reliable QIBs for use in the clinical imaging community. Some of QIBA's development of quantitative diffusion-weighted imaging and dynamic contrast-enhanced QIB profiles has been hampered by the lack of literature for repeatability and reproducibility of the derived QIBs. The available research on this topic is scant and is not in sync with improvements or upgrades in MRI technology over the years. This review focuses on the need for QIBs in oncology applications and emphasizes the importance of the assessment of their reproducibility and repeatability. Level of Evidence: 5 Technical Efficacy Stage: 1 J. Magn. Reson. Imaging 2019;49:e101-e121.
Keywords: DCE; DWI; MRI; quantitative imaging biomarkers.
© 2018 International Society for Magnetic Resonance in Medicine.
Figures


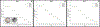


References
-
- Tofts P Quantitative MRI of the Brain Measuring Changes Caused by Disease Introduction. Quantitative Mri of the Brain: Measuring Changes Caused by Disease 2003:Xv–Xvi.
-
- Padhani AR, Khan AA. Diffusion-weighted (DW) and dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) for monitoring anticancer therapy. Target Oncol 2010;5(1):39–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA190299/CA/NCI NIH HHS/United States
- KL2 TR000131/TR/NCATS NIH HHS/United States
- P30 CA006973/CA/NCI NIH HHS/United States
- R01 CA148708/CA/NCI NIH HHS/United States
- HHSN268201500021C/HL/NHLBI NIH HHS/United States
- L30 CA209248/CA/NCI NIH HHS/United States
- HHSN268201300071C/EB/NIBIB NIH HHS/United States
- U01 CA154602/CA/NCI NIH HHS/United States
- UL1 TR001855/TR/NCATS NIH HHS/United States
- P01 CA085878/CA/NCI NIH HHS/United States
- U01 CA211205/CA/NCI NIH HHS/United States
- U01 CA140204/CA/NCI NIH HHS/United States
- P30 CA013148/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- HHSN268201000050C/EB/NIBIB NIH HHS/United States
- U01 CA166104/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

