Fibroblast growth factor 23 and α-Klotho co-dependent and independent functions
- PMID: 30451736
- PMCID: PMC6258326
- DOI: 10.1097/MNH.0000000000000467
Fibroblast growth factor 23 and α-Klotho co-dependent and independent functions
Abstract
Purpose of review: The current review examines what is known about the FGF-23/α-Klotho co-dependent and independent pathophysiological effects, and whether FGF-23 and/or α-Klotho are potential therapeutic targets.
Recent findings: FGF-23 is a hormone derived mainly from bone, and α-Klotho is a transmembrane protein. Together they form a trimeric signaling complex with FGFRs in target tissues to mediate the physiological functions of FGF-23. Local and systemic factors control FGF-23 release from osteoblast/osteocytes in bone, and circulating FGF-23 activates FGFR/α-Klotho complexes in kidney proximal and distal renal tubules to regulate renal phosphate excretion, 1,25 (OH)2D metabolism, sodium and calcium reabsorption, and ACE2 and α-Klotho expression. The resulting bone-renal-cardiac-immune networks provide a new understanding of bone and mineral homeostasis, as well as identify other biological effects FGF-23. Direct FGF-23 activation of FGFRs in the absence of α-Klotho is proposed to mediate cardiotoxic and adverse innate immune effects of excess FGF-23, particularly in chronic kidney disease, but this FGF-23, α-Klotho-independent signaling is controversial. In addition, circulating soluble Klotho (sKl) released from the distal tubule by ectodomain shedding is proposed to have beneficial health effects independent of FGF-23.
Summary: Separation of FGF-23 and α-Klotho independent functions has been difficult in mammalian systems and understanding FGF-23/α-Klotho co-dependent and independent effects are incomplete. Antagonism of FGF-23 is important in treatment of hypophosphatemic disorders caused by excess FGF-23, but its role in chronic kidney disease is uncertain. Administration of recombinant sKl is an unproven therapeutic strategy that theoretically could improve the healt span and lifespan of patients with α-Klotho deficiency.
Conflict of interest statement
Conflicts of interest
Dr. Quarles has received honoraria from Amgen for serving on a scientific advisory board.
Figures
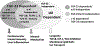



References
-
- Itoh N, Ornitz DM: Functional evolutionary history of the mouse fgf gene family. Dev Dyn (2008) 237(1):18–27. - PubMed
-
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical fgf receptor into a specific receptor for fgf23. Nature (2006) 444(7120):770–774. The seminial observations showing that α-Klotho is the obligate co-receptor required for FGF-23 activation of FGFRs in target tissues. - PubMed
-
- Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, Mohammadi M: Alpha-klotho is a non-enzymatic molecular scaffold for fgf23 hormone signalling. Nature (2018) 553(7689):461–466. This study provives the structural basis for formation of the FGF-23/α-Klotho/FGFR complexes, but overstates the conclusion regarding the “on demand” function of sKl. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

