Locomotion modulates specific functional cell types in the mouse visual thalamus
- PMID: 30451819
- PMCID: PMC6242985
- DOI: 10.1038/s41467-018-06780-3
Locomotion modulates specific functional cell types in the mouse visual thalamus
Abstract
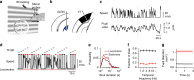
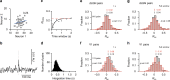
The visual system is composed of diverse cell types that encode distinct aspects of the visual scene and may form separate processing channels. Here we present further evidence for that hypothesis whereby functional cell groups in the dorsal lateral geniculate nucleus (dLGN) are differentially modulated during behavior. Using simultaneous multi-electrode recordings in dLGN and primary visual cortex (V1) of behaving mice, we characterized the impact of locomotor activity on response amplitude, variability, correlation and spatiotemporal tuning. Locomotion strongly impacts the amplitudes of dLGN and V1 responses but the effects on variability and correlations are relatively minor. With regards to tunings, locomotion enhances dLGN responses to high temporal frequencies, preferentially affecting ON transient cells and neurons with nonlinear responses to high spatial frequencies. Channel specific modulations may serve to highlight particular visual inputs during active behaviors.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

