Mavoglurant in Fragile X Syndrome: Results of two open-label, extension trials in adults and adolescents
- PMID: 30451888
- PMCID: PMC6242849
- DOI: 10.1038/s41598-018-34978-4
Mavoglurant in Fragile X Syndrome: Results of two open-label, extension trials in adults and adolescents
Abstract
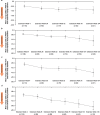
Fragile X syndrome (FXS) is the most common monogenic cause of inherited intellectual and developmental disabilities. Mavoglurant, a selective metabotropic glutamate receptor subtype-5 antagonist, has shown positive neuronal and behavioral effects in preclinical studies, but failed to demonstrate any behavioral benefits in two 12-week, randomized, placebo-controlled, double-blind, phase IIb studies in adults and adolescents with FXS. Here we report the long-term safety (primary endpoint) and efficacy (secondary endpoint) results of the open-label extensions. Adolescent (n = 119, aged 12-19 years) and adult (n = 148, aged 18-45 years) participants received up to 100 mg bid mavoglurant for up to 34 months. Both extension studies were terminated prematurely due to lack of proven efficacy in the core studies. Mavoglurant was well tolerated with no new safety signal. Five percent of adults and 16.9 percent of adolescents discontinued treatment due to adverse events. Gradual and consistent behavioral improvements as measured by the ABC-CFX scale were observed, which were numerically superior to those seen in the placebo arm of the core studies. These two extension studies confirm the long-term safety of mavoglurant in FXS, but further investigations are required to determine whether and under which conditions the significant preclinical results obtained with mGluR5 inhibition can translate to humans.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

