Selective inhibition of carbonic anhydrase IX over carbonic anhydrase XII in breast cancer cells using benzene sulfonamides: Disconnect between activity and growth inhibition
- PMID: 30452451
- PMCID: PMC6242694
- DOI: 10.1371/journal.pone.0207417
Selective inhibition of carbonic anhydrase IX over carbonic anhydrase XII in breast cancer cells using benzene sulfonamides: Disconnect between activity and growth inhibition
Retraction in
-
Retraction: Selective inhibition of carbonic anhydrase IX over carbonic anhydrase XII in breast cancer cells using benzene sulfonamides: Disconnect between activity and growth inhibition.PLoS One. 2025 Jun 18;20(6):e0326621. doi: 10.1371/journal.pone.0326621. eCollection 2025. PLoS One. 2025. PMID: 40531832 Free PMC article. No abstract available.
Abstract
Carbonic anhydrases (CAs) have been linked to tumor progression, particularly membrane-bound CA isoform IX (CA IX). The role of CA IX in the context of breast cancer is to regulate the pH of the tumor microenvironment. In contrast to CA IX, expression of CA XII, specifically in breast cancer, is associated with better outcome despite performing the same catalytic function. In this study, we have structurally modeled the orientation of bound ureido-substituted benzene sulfonamides (USBs) within the active site of CA XII, in comparison to CA IX and cytosolic off-target CA II, to understand isoform specific inhibition. This has identified specific residues within the CA active site, which differ between isoforms that are important for inhibitor binding and isoform specificity. The ability of these sulfonamides to block CA IX activity in breast cancer cells is less effective than their ability to block activity of the recombinant protein (by one to two orders of magnitude depending on the inhibitor). The same is true for CA XII activity but now they are two to three orders of magnitude less effective. Thus, there is significantly greater specificity for CA IX activity over CA XII. While the inhibitors block cell growth, without inducing cell death, this again occurs at two orders of magnitude above the Ki values for inhibition of CA IX and CA XII activity in their respective cell types. Surprisingly, the USBs inhibited cell growth even in cells where CA IX and CA XII expression was ablated. Despite the potential for these sulfonamides as chemotherapeutic agents, these data suggest that we reconsider the role of CA activity on growth potentiation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







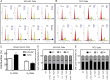
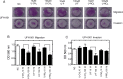
References
-
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–74. 10.1073/pnas.191367098 ; PubMed Central PMCID: PMCPMC58566. - DOI - PMC - PubMed
-
- Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–81. 10.1200/JCO.1999.17.5.1474 . - DOI - PubMed
-
- Abe O, Abe R, Enomoto K, Kikuchi K, Koyama H, Masuda H, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. WOS:000229082300022. 10.1016/S0140-6736(05)66544-0 - DOI - PubMed
-
- Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9(9):631–43. 10.1038/nrc2713 . - DOI - PubMed
-
- Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, et al. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14(24):8010–8. 10.1158/1078-0432.CCR-08-1208 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

