Completion Rate and Reporting of Mandatory Pediatric Postmarketing Studies Under the US Pediatric Research Equity Act
- PMID: 30452498
- PMCID: PMC6583440
- DOI: 10.1001/jamapediatrics.2018.3416
Completion Rate and Reporting of Mandatory Pediatric Postmarketing Studies Under the US Pediatric Research Equity Act
Abstract
Importance: Many medicines prescribed to children have not been studied or formally approved for pediatric use. The Pediatric Research Equity Act of 2003 authorized the US Food and Drug Administration (FDA) to require pediatric clinical studies.
Objective: To evaluate the characteristics, completion rate, and transparency of study design and results for mandatory pediatric postmarketing studies required under the Pediatric Research Equity Act.
Design and setting: A retrospective cohort study was conducted of pediatric postmarketing studies required for new drugs and new indications approved by the FDA between January 1, 2007, and December 31, 2014, with follow-up through December 1, 2017. Information on the status, design, and results of pediatric studies was obtained from publicly available FDA databases and ClinicalTrials.gov, direct communication with the FDA, and searches of MEDLINE, EMBASE, and Web of Science for peer-reviewed publications.
Main outcomes and measures: Characteristics and transparency of pediatric studies, results reporting (in ClinicalTrials.gov, peer-reviewed literature, or FDA documents), and availability of pediatric information in drug labels. Rates and times to study completion were evaluated using Cox proportional hazards regression models.
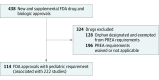
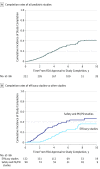
Results: Between 2007 and 2014, the FDA approved 114 new drugs and new indications for already approved drugs that were subject to Pediatric Research Equity Act requirements. These drugs were associated with 222 required pediatric postmarketing clinical studies. Overall, 75 pediatric studies (33.8%) were completed as of December 1, 2017. The rates of completion were significantly lower for efficacy studies (38 of 132 [28.8%]) compared with pharmacokinetic studies (19 of 34 [55.9%]; adjusted hazard ratio, 0.31; 95% CI, 0.12-0.82). Information on randomization, blinding, comparator, end point, and study size could not be identified for 74 studies (33.3%), and no reason for discontinuation was provided for 29 of the 42 discontinued studies (69.0%). Among the completed studies, the results were reported for 57 (76.0%). At the time of approval, 18 of 114 drug approvals (15.8%) had any pediatric efficacy, safety, or dosing information in their labels. After a median duration of follow-up of 6.8 years (interquartile range, 4.7-9.1 years), 47 of 114 of drug labels (41.2%) had any pediatric information.
Conclusions and relevance: Only 33.8% of mandatory pediatric postmarketing studies have been completed after a median follow-up of 6.8 years, and most drug labels do not include information important for pediatric use. To improve evidence-based prescribing of medicines to children, more timely completion of pediatric drug studies is needed.
Conflict of interest statement
Figures
Comment in
-
Development of Therapeutics for Children-A Tricky Balancing Act.JAMA Pediatr. 2019 Jan 1;173(1):18-19. doi: 10.1001/jamapediatrics.2018.4026. JAMA Pediatr. 2019. PMID: 30452501 No abstract available.
References
-
- National Academy of Medicine (formerly Institute of Medicine) Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC: The National Academies Press; 2012. - PubMed

