Duration of Pediatric Clinical Trials Submitted to the US Food and Drug Administration
- PMID: 30452504
- PMCID: PMC6526087
- DOI: 10.1001/jamapediatrics.2018.3227
Duration of Pediatric Clinical Trials Submitted to the US Food and Drug Administration
Abstract
Importance: The increasing prevalence of pediatric chronic disease has resulted in increased exposure to long-term drug therapy in children. The duration of recently completed drug trials that support approval for drug therapy in children with chronic diseases has not been systematically evaluated. Such information is a vital first step in forming safety pharmacovigilance strategies for drugs used for long-term therapy in children.
Objective: To characterize the duration of clinical trials submitted to the US Food and Drug Administration (FDA) for pediatric drug approvals, with a focus on drugs used for long-term therapy.
Design and setting: A review was performed of all safety and efficacy clinical trials conducted under the Best Pharmaceuticals for Children Act or the Pediatric Research Equity Act and submitted to the FDA from September 1, 2007, to December 31, 2014, to support the approval of drugs frequently used for long-term therapy in children. Statistical analysis was performed from July 1, 2015, to December 31, 2017.
Main outcomes and measures: Maximum duration of trials submitted to support FDA approval of drugs for children.
Results: A total of 306 trials supporting 86 drugs intended for long-term use in children were eligible for the primary analysis. The drugs most commonly evaluated were for treatment of neurologic (25 [29%]), pulmonary (16 [19%]), and anti-infective (14 [16%]) indications. The median maximum trial duration by drug was 44 weeks (minimum, 1.1 week; maximum, 364 weeks). For nearly two-thirds of the drugs (52 [61%]), the maximum trial duration was less than 52 weeks. For 10 of the drugs (12%), the maximum trial duration was 3 years or more. Maximum duration of trials did not vary by therapeutic category, minimum age of enrollment, calendar year, or legislative mandate.
Conclusions and relevance: Pediatric clinical trials designed to sufficiently investigate drug safety and efficacy to support FDA approval are of relatively limited duration. Given the potential long-term exposure of patients to these drugs, the clinical community should consider whether new approaches are needed to better understand the safety associated with long-term use of these drugs.
Conflict of interest statement
Conflict of Interest Disclosures:
Dr Smith reported receiving compensation for serving as a consultant for Astellas Pharma, Lediant, and Nestec. No other disclosures were reported.
Figures

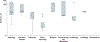

Comment in
-
Development of Therapeutics for Children-A Tricky Balancing Act.JAMA Pediatr. 2019 Jan 1;173(1):18-19. doi: 10.1001/jamapediatrics.2018.4026. JAMA Pediatr. 2019. PMID: 30452501 No abstract available.

