Corneal pain and experimental model development
- PMID: 30453079
- PMCID: PMC6690397
- DOI: 10.1016/j.preteyeres.2018.11.005
Corneal pain and experimental model development
Abstract
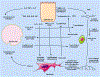
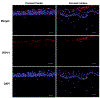
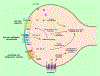
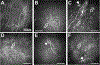
The cornea is a valuable tissue for studying peripheral sensory nerve structure and regeneration due to its avascularity, transparency, and dense innervation. Somatosensory innervation of the cornea serves to identify changes in environmental stimuli at the ocular surface, thereby promoting barrier function to protect the eye against injury or infection. Due to regulatory demands to screen ocular safety of potential chemical exposure, a need remains to develop functional human tissue models to predict ocular damage and pain using in vitro-based systems to increase throughput and minimize animal use. In this review, we summarize the anatomical and functional roles of corneal innervation in propagation of sensory input, corneal neuropathies associated with pain, and the status of current in vivo and in vitro models. Emphasis is placed on tissue engineering approaches to study the human corneal pain response in vitro with integration of proper cell types, controlled microenvironment, and high-throughput readouts to predict pain induction. Further developments in this field will aid in defining molecular signatures to distinguish acute and chronic pain triggers based on the immune response and epithelial, stromal, and neuronal interactions that occur at the ocular surface that lead to functional outcomes in the brain depending on severity and persistence of the stimulus.
Keywords: Cornea; Dry eye; Neuropeptides; Nociception; Pain; Tissue engineering.
Copyright © 2018. Published by Elsevier Ltd.
Conflict of interest statement
Figures





References
-
- 2007. Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5, 179–193. - PubMed
-
- Acera A, Rocha G, Vecino E, Lema I, Durán JA, 2008. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic research 40, 315–321. - PubMed
-
- Acosta MC, Luna C, Quirce S, Belmonte C, Gallar J, 2013. Changes in sensory activity of ocular surface sensory nerves during allergic keratoconjunctivitis. Pain 154, 2353–2362. - PubMed
-
- Adriaens E, Barroso J, Eskes C, Hoffmann S, McNamee P, Alepee N, Bessou-Touya S, De Smedt A, De Wever B, Pfannenbecker U, Tailhardat M, Zuang V, 2014. Retrospective analysis of the Draize test for serious eye damage/eye irritation: importance of understanding the in vivo endpoints under UN GHS/EU CLP for the development and evaluation of in vitro test methods. Archives of toxicology 88, 701–723. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

