Vacuolar Proton Pyrophosphatase Is Required for High Magnesium Tolerance in Arabidopsis
- PMID: 30453498
- PMCID: PMC6274811
- DOI: 10.3390/ijms19113617
Vacuolar Proton Pyrophosphatase Is Required for High Magnesium Tolerance in Arabidopsis
Abstract
Magnesium (Mg2+) is an essential nutrient in all organisms. However, high levels of Mg2+ in the environment are toxic to plants. In this study, we identified the vacuolar-type H⁺-pyrophosphatase, AVP1, as a critical enzyme for optimal plant growth under high-Mg conditions. The Arabidopsis avp1 mutants displayed severe growth retardation, as compared to the wild-type plants upon excessive Mg2+. Unexpectedly, the avp1 mutant plants retained similar Mg content to wild-type plants under either normal or high Mg conditions, suggesting that AVP1 may not directly contribute to Mg2+ homeostasis in plant cells. Further analyses confirmed that the avp1 mutant plants contained a higher pyrophosphate (PPi) content than wild type, coupled with impaired vacuolar H⁺-pyrophosphatase activity. Interestingly, expression of the Saccharomyces cerevisiae cytosolic inorganic pyrophosphatase1 gene IPP1, which facilitates PPi hydrolysis but not proton translocation into vacuole, rescued the growth defects of avp1 mutants under high-Mg conditions. These results provide evidence that high-Mg sensitivity in avp1 mutants possibly resulted from elevated level of cytosolic PPi. Moreover, genetic analysis indicated that mutation of AVP1 was additive to the defects in mgt6 and cbl2 cbl3 mutants that are previously known to be impaired in Mg2+ homeostasis. Taken together, our results suggest AVP1 is required for cellular PPi homeostasis that in turn contributes to high-Mg tolerance in plant cells.
Keywords: AtAVP1; cellular PPi homeostasis; high-Mg tolerance; vacuolar H+-pyrophosphatase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


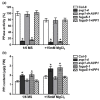
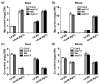


References
-
- George G.M., van der Merwe M.J., Nunes-Nesi A., Bauer R., Fernie A.R., Kossmann J., Lloyd J.R. Virus-induced gene silencing of plastidial soluble inorganic pyrophosphatase impairs essential leaf anabolic pathways and reduced drought stress tolerance in Nicotiana benthamiana. Plant Physiol. 2010;154:55–66. doi: 10.1104/pp.110.157776. - DOI - PMC - PubMed
-
- Lόpez-Marqués R.L., Pérez-Castiñeira J.R., Losada M., Serrano A. Differential regulation of soluble and membrane-bound inorganic pyrophosphatases in the photosynthetic bacterium Rhodospirillum rubrum provides insights into pyrophosphate-based stress bioenergetics. J. Bacteriol. 2004;186:5418–5426. doi: 10.1128/JB.186.16.5418-5426.2004. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

