UBE2E1 Is Preferentially Expressed in the Cytoplasm of Slow-Twitch Fibers and Protects Skeletal Muscles from Exacerbated Atrophy upon Dexamethasone Treatment
- PMID: 30453501
- PMCID: PMC6262581
- DOI: 10.3390/cells7110214
UBE2E1 Is Preferentially Expressed in the Cytoplasm of Slow-Twitch Fibers and Protects Skeletal Muscles from Exacerbated Atrophy upon Dexamethasone Treatment
Erratum in
-
Erratum: Polge, C., et al. UBE2E1 Is Preferentially Expressed in the Cytoplasm of Slow-Twitch Fibers and Protects Skeletal Muscles from Exacerbated Atrophy upon Dexamethasone Treatment. Cells 2018, 7, 214.Cells. 2018 Dec 4;7(12):242. doi: 10.3390/cells7120242. Cells. 2018. PMID: 30518141 Free PMC article.
Abstract
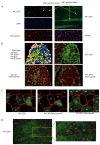
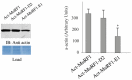
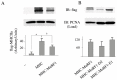
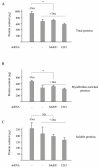
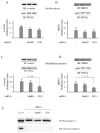
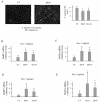
Skeletal muscle mass is reduced during many diseases or physiological situations (disuse, aging), which results in decreased strength and increased mortality. Muscle mass is mainly controlled by the ubiquitin-proteasome system (UPS), involving hundreds of ubiquitinating enzymes (E2s and E3s) that target their dedicated substrates for subsequent degradation. We recently demonstrated that MuRF1, an E3 ubiquitin ligase known to bind to sarcomeric proteins (telethonin, α-actin, myosins) during catabolic situations, interacts with 5 different E2 enzymes and that these E2-MuRF1 couples are able to target telethonin, a small sarcomeric protein, for degradation. Amongst the E2s interacting with MuRF1, E2E1 was peculiar as the presence of the substrate was necessary for optimal MuRF1-E2E1 interaction. In this work, we focused on the putative role of E2E1 during skeletal muscle atrophy. We found that E2E1 expression was restricted to type I and type IIA muscle fibers and was not detectable in type IIB fibers. This strongly suggests that E2E1 targets are fiber-specific and may be strongly linked to the contractile and metabolic properties of the skeletal muscle. However, E2E1 knockdown was not sufficient for preserving the protein content in C2C12 myotubes subjected to a catabolic state (dexamethasone treatment), suggesting that E2E1 is not involved in the development of muscle atrophy. By contrast, E2E1 knockdown aggravated the atrophying process in both catabolic C2C12 myotubes and the Tibialis anterior muscle of mice, suggesting that E2E1 has a protective effect on muscle mass.
Keywords: E2 ubiquitin-conjugating enzymes; E3 ubiquitin ligases; MuRF1; UBE2E1; actin; atrophy; myosin heavy chain; skeletal muscle; ubiquitin-proteasome system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fearon K., Arends J., Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nature reviews. Clin. Oncol. 2013;10:90–99. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

