Human Fetal Astrocytes Infected with Zika Virus Exhibit Delayed Apoptosis and Resistance to Interferon: Implications for Persistence
- PMID: 30453621
- PMCID: PMC6266559
- DOI: 10.3390/v10110646
Human Fetal Astrocytes Infected with Zika Virus Exhibit Delayed Apoptosis and Resistance to Interferon: Implications for Persistence
Abstract
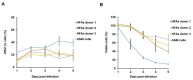
Zika virus (ZIKV) infection and persistence during pregnancy can lead to microcephaly and other fetal neurological disorders collectively known as Congenital Zika Syndrome. The immunological and virological events that contribute to the establishment of persistent ZIKV infection in humans are unclear though. Here we show that human fetal astrocytes (HFAs), the most abundant cell type in the central nervous system, become persistently infected with ZIKV resulting in continuous viral shedding for at least one month; a process that is facilitated by TIM/TAM receptors. HFAs are relatively resistant to ZIKV-induced apoptosis, a factor that may be important for chronic infection of these cells. Once infection was established, interferon treatment did not reduce virus replication. Moreover, the fact that the innate immune system was highly activated in persistently infected HFAs indicates that the virus can thrive in the presence of a sustained antiviral response. RNAseq analyses of persistently infected cells revealed that ZIKV alters host gene expression in a manner that could affect developmental processes. Conversely, data from sequencing of ZIKV genomes in persistently infected HFAs suggest that adaptive mutations were not required for establishing chronic infection. Based on these results, we postulate that HFAs are reservoirs for ZIKV in the fetal brain and that moderate apoptosis combined with inefficient antiviral response from these cells may contribute to the establishment of chronic brain infection associated with the ZIKV neurodevelopmental abnormalities.
Keywords: Zika virus; apoptosis; astrocytes; interferon; persistence.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







References
-
- Brasil P., Pereira J.P., Jr., Moreira M.E., Ribeiro Nogueira R.M., Damasceno L., Wakimoto M., Rabello R.S., Valderramos S.G., Halai U.A., Salles T.S., et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. - DOI - PMC - PubMed
-
- Franca G.V., Schuler-Faccini L., Oliveira W.K., Henriques C.M., Carmo E.H., Pedi V.D., Nunes M.L., Castro M.C., Serruya S., Silveira M.F., et al. Congenital Zika virus syndrome in Brazil: A case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388:891–897. doi: 10.1016/S0140-6736(16)30902-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

