Willingness to Participate and Associated Factors in a Zika Vaccine Trial in Indonesia: A Cross-Sectional Study
- PMID: 30453663
- PMCID: PMC6266114
- DOI: 10.3390/v10110648
Willingness to Participate and Associated Factors in a Zika Vaccine Trial in Indonesia: A Cross-Sectional Study
Abstract
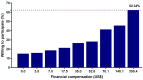
One of the crucial steps during trials for Zika and other vaccines is to recruit participants and to understand how participants' attitudes and sociodemographic characteristics affect willingness to participate (WTP). This study was conducted to assess WTP, its explanatory variables, and the impact of financial compensation on WTP in Indonesia. A health facility-based cross-sectional study was conducted in eleven regencies in the Aceh and West Sumatra provinces of Indonesia. Participants were recruited via a convenience sampling method and were interviewed. The associations between explanatory variables and WTP were assessed using a two-step logistic regression analysis. A total of 1,102 parents were approached, and of these 956 (86.8%) completed the interview and were included in analysis. Of those, 144 (15.1%) were willing to participate in a Zika vaccine trial without a financial compensation. In the multivariate analysis, WTP was tied to an age of more than 50 years old, compared to 20⁻29 years (odds ratio (OR): 5.0; 95% confidence interval (CI): 2.37⁻10.53), to being female (OR: 2.20; 95% CI: 1.11⁻4.37), and to having heard about Zika (OR: 2.41; 95% CI: 1.59⁻3.65). Participants' WTP increased gradually with higher financial compensation. The rate of WTP increased to 62.3% at the highest offer (US$ 350.4), and those who were still unwilling to participate (37.7%) had a poorer attitude towards childhood vaccination. This study highlights that pre-existing knowledge about Zika and attitudes towards childhood vaccination are important in determining community members being willing to participate in a vaccine trial. Financial incentives are still an important factor to enhance participant recruitment during a vaccine trial.
Keywords: Zika; Zika vaccine; vaccine acceptance; vaccine trial; willingness to participate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization (WHO) Zika Situation Report. [(accessed on 1 January 2018)]; Available online: http://www.who.int/emergencies/zika-virus/situation-report/10-march-2017...
-
- Cauchemez S., Besnard M., Bompard P., Dub T., Guillemette-Artur P., Eyrolle-Guignot D., Salje H., Van Kerkhove M.D., Abadie V., Garel C., et al. Association between Zika virus and microcephaly in French Polynesia, 2013–2015: A retrospective study. Lancet. 2016;387:2125–2132. doi: 10.1016/S0140-6736(16)00651-6. - DOI - PMC - PubMed
-
- De Araujo T.V.B., Rodrigues L.C., Ximenes R.A.D.A., Miranda-Filho D.D.B., Montarroyos U.R., de Melo A.P.L., Valongueiro S., de Albuquerque M.D.F.P.M., Souza W.V., Braga C., et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: Preliminary report of a case-control study. Lancet Infect. Dis. 2016;16:1356–1363. doi: 10.1016/S1473-3099(16)30318-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

