Protein Structural Dynamics of Wild-Type and Mutant Homodimeric Hemoglobin Studied by Time-Resolved X-Ray Solution Scattering
- PMID: 30453670
- PMCID: PMC6274816
- DOI: 10.3390/ijms19113633
Protein Structural Dynamics of Wild-Type and Mutant Homodimeric Hemoglobin Studied by Time-Resolved X-Ray Solution Scattering
Abstract
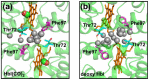
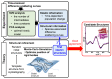
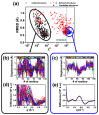
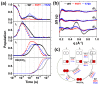
The quaternary transition between the relaxed (R) and tense (T) states of heme-binding proteins is a textbook example for the allosteric structural transition. Homodimeric hemoglobin (HbI) from Scapharca inaequivalvis is a useful model system for investigating the allosteric behavior because of the relatively simple quaternary structure. To understand the cooperative transition of HbI, wild-type and mutants of HbI have been studied by using time-resolved X-ray solution scattering (TRXSS), which is sensitive to the conformational changes. Herein, we review the structural dynamics of HbI investigated by TRXSS and compare the results of TRXSS with those of other techniques.
Keywords: allostery; homodimeric hemoglobin; molecular cooperativity; protein dynamics; time-resolved X-ray solution scattering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Direct observation of cooperative protein structural dynamics of homodimeric hemoglobin from 100 ps to 10 ms with pump-probe X-ray solution scattering.J Am Chem Soc. 2012 Apr 25;134(16):7001-8. doi: 10.1021/ja210856v. Epub 2012 Apr 12. J Am Chem Soc. 2012. PMID: 22494177 Free PMC article.
-
Protein structural dynamics revealed by time-resolved X-ray solution scattering.Acc Chem Res. 2015 Aug 18;48(8):2200-8. doi: 10.1021/acs.accounts.5b00198. Epub 2015 Jul 2. Acc Chem Res. 2015. PMID: 26134248 Free PMC article.
-
Structure of the Fe-heme in the hemodimeric hemoglobin from Scapharca inaequivalvis and in the T721 mutant: an X-ray absorption spectroscopic study at low temperature.Eur Biophys J. 2001;29(8):559-68. doi: 10.1007/s002490000102. Eur Biophys J. 2001. PMID: 11288830
-
Structural and thermodynamic aspects of cooperativity in the homodimeric hemoglobin from Scapharca inaequivalvis.Biophys Chem. 2000 Aug 30;86(2-3):173-8. doi: 10.1016/s0301-4622(00)00162-9. Biophys Chem. 2000. PMID: 11026682 Review.
-
Allosteric transitions in hemoglobin revisited.Biochim Biophys Acta Gen Subj. 2020 Feb;1864(2):129335. doi: 10.1016/j.bbagen.2019.03.021. Epub 2019 Apr 2. Biochim Biophys Acta Gen Subj. 2020. PMID: 30951803 Review.
Cited by
-
Structural Dynamics of C2F4I2 in Cyclohexane Studied via Time-Resolved X-ray Liquidography.Int J Mol Sci. 2021 Sep 10;22(18):9793. doi: 10.3390/ijms22189793. Int J Mol Sci. 2021. PMID: 34575954 Free PMC article.
-
Effect of the abolition of intersubunit salt bridges on allosteric protein structural dynamics.Chem Sci. 2021 May 10;12(23):8207-8217. doi: 10.1039/d1sc01207j. Chem Sci. 2021. PMID: 34194711 Free PMC article.
-
Design of Aromatic Interaction Networks in a Protein Cage Modulated by Fluorescent Ligand Binding.Adv Sci (Weinh). 2025 Apr;12(15):e2417030. doi: 10.1002/advs.202417030. Epub 2025 Feb 20. Adv Sci (Weinh). 2025. PMID: 39973762 Free PMC article.
-
Reversible molecular motional switch based on circular photoactive protein oligomers exhibits unexpected photo-induced contraction.Cell Rep Phys Sci. 2021 Aug 18;2(8):100512. doi: 10.1016/j.xcrp.2021.100512. Epub 2021 Jul 22. Cell Rep Phys Sci. 2021. PMID: 35509376 Free PMC article.
-
Ultrafast coherent motion and helix rearrangement of homodimeric hemoglobin visualized with femtosecond X-ray solution scattering.Nat Commun. 2021 Jun 16;12(1):3677. doi: 10.1038/s41467-021-23947-7. Nat Commun. 2021. PMID: 34135339 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

