Production of Omegas-6 and 9 from the Hydrolysis of Açaí and Buriti Oils by Lipase Immobilized on a Hydrophobic Support
- PMID: 30453683
- PMCID: PMC6278552
- DOI: 10.3390/molecules23113015
Production of Omegas-6 and 9 from the Hydrolysis of Açaí and Buriti Oils by Lipase Immobilized on a Hydrophobic Support
Abstract
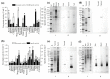
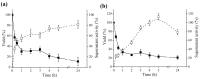
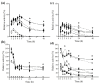
This paper describes a bioprocess to obtain omegas-6 and 9 from the hydrolysis of Açaí (Euterpe oleracea Martius) and Buriti (Mauritia flexuosa) oils by lipases immobilized on octyl-sepharose. For this, oils and butters were initially selected as the carbon source which resulted in higher production of lipases in Beauveria bassiana and Fusarium oxysporum cultures. The carbon source that provided secretion of lipase by B. bassiana was Açaí oil, and for F. oxysporum, Bacuri butter. Lipases obtained under these conditions were immobilized on octyl-sepharose, and both, the derivatives and the crude extracts were biochemically characterized. It was observed that the immobilization promoted an increase of stability in B. bassiana and F. oxysporum lipase activities at the given temperatures and pH. In addition, the immobilization promoted hyperactivation of B. bassiana and F. oxysporum lipase activities being 23.5 and 11.0 higher than free enzyme, respectively. The hydrolysis of Açaí and Buriti oils by the derivatives was done in a biphasic (organic/aqueous) system, and the products were quantified in RP-HPLC. The results showed the potential of these immobilized lipases to obtain omegas-6 and 9 from Brazilian natural oils. This work may improve the enzymatic methodologies for obtaining foods and drugs enriched with fatty acids.
Keywords: Açaí (Euterpe oleracea Martius); Beauveria bassiana; Buriti (Mauritia flexuosa); Fusarium oxysporum; immobilization; lipase; octyl-sepharose; omega-6; omega-9.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Zielińska A., Nowak I. Fatty Acids in Vegetable Oils and Their Importance in Cosmetic Industry. Chemik. 2014;68:103–110.
-
- Bhardwaj K., Verma N., Trivedi R.K., Bhardwaj S., Shukla N. Significance of Ratio of Omega-3 and Omega-6 in Human Health with Special Reference to Flaxseed Oil. Int. J. Biol. Chem. 2016;10:1–6. doi: 10.3923/ijbc.2016.1.6. - DOI
-
- Omega-3, 6, and 9 and How They Add Up. [(accessed on 5 October 2018)]; Available online: https://www.uccs.edu/healthcircle/sites/healthcircle/files/inline-files/....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

