Long-term performance of an external stent for saphenous vein grafts: the VEST IV trial
- PMID: 30453984
- PMCID: PMC6245530
- DOI: 10.1186/s13019-018-0803-9
Long-term performance of an external stent for saphenous vein grafts: the VEST IV trial
Abstract
Background: Externally stenting saphenous vein grafts reduces intimal hyperplasia, improves lumen uniformity and reduces oscillatory shear stress 1 year following surgery. The present study is the first to present the longer-term (4.5 years) performance and biomechanical effects of externally stented saphenous vein grafts.
Methods: Thirty patients previously implanted with the VEST external stent in the randomized, within-patient-controlled VEST I study were followed up for adverse events; 21 of these were available to undergo coronary angiography and intravascular ultrasound.
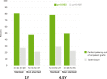
Results: Twenty-one stented and 29 nonstented saphenous vein grafts were evaluated by angiography and ultrasound at 4.5 ± 0.3 years. Vein graft failure rates were comparable between stented and nonstented grafts (30 and 23% respectively; p = 0.42). All failures were apparent at 1 year except for one additional nonstented failure at 4.5 years. In patent vein grafts, Fitzgibbon perfect patency remained significantly higher in the stented versus nonstented vein grafts (81 and 48% respectively, p = 0.002), while intimal hyperplasia area (4.27 mm2 ± 1.27 mm2 and 5.23 mm2 ± 1.83 mm2 respectively, p < 0.001) and thickness (0.36 mm ± 0.09 mm and 0.42 mm ± 0.11 mm respectively, p < 0.001) were significantly reduced. Intimal hyperplasia proliferation correlated with lumen uniformity and with the distance between the stent and the lumen (p = 0.04 and p < 0.001 respectively).
Conclusions: External stenting mitigates saphenous vein graft remodeling and significantly reduces diffuse intimal hyperplasia and the development of lumen irregularities 4.5 years after coronary artery bypass surgery. Close conformity of the stent to the vessel wall appears to be an important factor.
Trial registration: NCT01415245 . Registered 11 August 2011.
Keywords: Coronary artery bypass graft surgery; External stent; Intimal hyperplasia; Saphenous vein graft.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol conformed to the Declaration of Helsinki and was approved by a UK Research Ethics Committee (NRES Committee London – City Road & Hampstead, REC reference 15/LO/1862). Each patient gave their informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
Prof Taggart serves as a consultant to Vascular Graft Solutions, has stock ownership and receives consulting fees. Prof Taggart has received travel support and speaking honoraria from Vascular Graft Solutions. The remaining authors have no disclosures relating to this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.24.3108. - DOI - PubMed

