Active full-length DNA Aβ42 immunization in 3xTg-AD mice reduces not only amyloid deposition but also tau pathology
- PMID: 30454039
- PMCID: PMC6245829
- DOI: 10.1186/s13195-018-0441-4
Active full-length DNA Aβ42 immunization in 3xTg-AD mice reduces not only amyloid deposition but also tau pathology
Abstract
Background: Alzheimer's disease (AD) is the most well-known and most common type of age-related dementia. Amyloid deposition and hyperphosphorylation of tau protein are both pathological hallmarks of AD. Using a triple-transgenic mouse model (3xTg-AD) that develops plaques and tangles in the brain similar to human AD, we provide evidence that active full-length DNA amyloid-β peptide 1-42 (Aβ42) trimer immunization leads to reduction of both amyloid and tau aggregation and accumulation.
Methods: Immune responses were monitored by enzyme-linked immunosorbent assay (ELISA) (antibody production) and enzyme-linked immunospot (cellular activation, cytokine production). Brains from 20-month-old 3x Tg-AD mice that had received DNA Aβ42 immunotherapy were compared with brains from age- and gender-matched transgenic Aβ42 peptide-immunized and control mice by histology, Western blot analysis, and ELISA. Protein kinase activation and kinase levels were studied in Western blots from mouse hemibrain lysates.
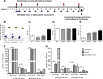
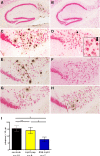
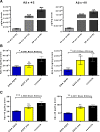
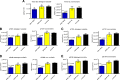
Results: Quantitative ELISA showed a 40% reduction of Aβ42 peptide and a 25-50% reduction of total tau and different phosphorylated tau molecules in the DNA Aβ42 trimer-immunized 3xTg-AD mice compared with nonimmunized 3xTg-AD control animals. Plaque and Aβ peptide reductions in the brain were due to the anti-Aβ antibodies generated following the immunizations. Reductions of tau were likely due to indirect actions such as less Aβ in the brain resulting in less tau kinase activation.
Conclusions: The significance of these findings is that DNA Aβ42 trimer immunotherapy targets two major pathologies in AD-amyloid plaques and neurofibrillary tangles-in one vaccine without inducing inflammatory T-cell responses, which carry the danger of autoimmune inflammation, as found in a clinical trial using active Aβ42 peptide immunization in patients with AD (AN1792).
Keywords: Alzheimer’s disease; Amyloid-β; Aβ oligomer; DNA vaccination; Immunotherapy; Tau; Tau kinases.
Conflict of interest statement
Ethics approval
Animal use for this study was approved by the Institutional Animal Care and Use Committee at UTSW
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.WNL.0000073623.84147.A8. - DOI - PubMed
-
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM, AN1792(QS-21)-201 Study Team Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2006;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. - DOI - PubMed
-
- Blennow K, Zetterberg H, Rinne JO, Salloway S, Wei J, Black R, Grundman M, Liu E, AAB-001 201/202 Investigators Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol. 2011;69(8):1002–1010. - PubMed
-
- Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, Piorkowska K, Gafner V, Atwal JK, Maloney J, Chen M, Gogineni A, Weimer RM, Mortensen DL, Friesenhahn M, Ho C, Paul R, Pfeifer A, Muhs A, Watts RJ. An effector-reduced anti-β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J Neurosci. 2012;32(28):9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

