Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC
- PMID: 30454647
- PMCID: PMC6242467
- DOI: 10.1016/j.cell.2018.09.053
Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC
Abstract
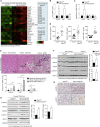
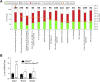
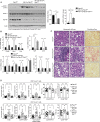
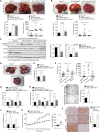
Obesity is a major driver of cancer, especially hepatocellular carcinoma (HCC). The prevailing view is that non-alcoholic steatohepatitis (NASH) and fibrosis or cirrhosis are required for HCC in obesity. Here, we report that NASH and fibrosis and HCC in obesity can be dissociated. We show that the oxidative hepatic environment in obesity inactivates the STAT-1 and STAT-3 phosphatase T cell protein tyrosine phosphatase (TCPTP) and increases STAT-1 and STAT-3 signaling. TCPTP deletion in hepatocytes promoted T cell recruitment and ensuing NASH and fibrosis as well as HCC in obese C57BL/6 mice that normally do not develop NASH and fibrosis or HCC. Attenuating the enhanced STAT-1 signaling prevented T cell recruitment and NASH and fibrosis but did not prevent HCC. By contrast, correcting STAT-3 signaling prevented HCC without affecting NASH and fibrosis. TCPTP-deletion in hepatocytes also markedly accelerated HCC in mice treated with a chemical carcinogen that promotes HCC without NASH and fibrosis. Our studies reveal how obesity-associated hepatic oxidative stress can independently contribute to the pathogenesis of NASH, fibrosis, and HCC.
Keywords: PTPN2; STAT-1; STAT-3; T cells; fibrosis; hepatocellular carcinoma; non-alcoholic steatohepatitis; nonalcoholic fatty liver disease; obesity; oxidative stress; protein tyrosine phosphatase.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures









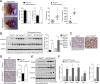
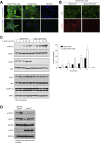
Comment in
-
New pathways in development of liver cancer.Nat Rev Endocrinol. 2018 Dec;15(1):2. doi: 10.1038/s41574-018-0129-7. Nat Rev Endocrinol. 2018. PMID: 30425341 No abstract available.
-
New pathways in development of liver cancer.Nat Rev Gastroenterol Hepatol. 2018 Dec;15(12):718. doi: 10.1038/s41575-018-0086-6. Nat Rev Gastroenterol Hepatol. 2018. PMID: 30429585 No abstract available.
-
Dividing paths in fatty liver disease.Nat Rev Cancer. 2019 Jan;19(1):5. doi: 10.1038/s41568-018-0086-4. Nat Rev Cancer. 2019. PMID: 30451985 No abstract available.
-
NASH and HCC Are Driven by Different Signaling Pathways with a Common Regulator.Cell Metab. 2019 Jan 8;29(1):3-4. doi: 10.1016/j.cmet.2018.12.012. Cell Metab. 2019. PMID: 30625307
-
Molecular pathways between obesity, non-alcoholic steatohepatitis (NASH) and hepatocellular carcinoma (HCC).Hepatobiliary Surg Nutr. 2019 Aug;8(4):395-397. doi: 10.21037/hbsn.2019.03.13. Hepatobiliary Surg Nutr. 2019. PMID: 31489312 Free PMC article. No abstract available.
-
Signal transducer and activator of transcriptions (STATs)-at the crossroads of obesity-linked non-alcoholic steatohepatitis and hepatocellular carcinoma.Hepatobiliary Surg Nutr. 2019 Aug;8(4):407-410. doi: 10.21037/hbsn.2019.03.21. Hepatobiliary Surg Nutr. 2019. PMID: 31489316 Free PMC article. No abstract available.
-
Dissociating nonalcoholic steatohepatitis from hepatocellular carcinoma in obesity.Hepatobiliary Surg Nutr. 2020 Feb;9(1):73-76. doi: 10.21037/hbsn.2019.07.18. Hepatobiliary Surg Nutr. 2020. PMID: 32140483 Free PMC article. No abstract available.
-
Independent regulation of tumorigenesis and fibrosis in non-alcoholic fatty liver disease.Hepatobiliary Surg Nutr. 2020 Feb;9(1):106-108. doi: 10.21037/hbsn.2019.08.11. Hepatobiliary Surg Nutr. 2020. PMID: 32140493 Free PMC article. No abstract available.
References
-
- Alexander J., Torbenson M., Wu T.T., Yeh M.M. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J. Gastroenterol. Hepatol. 2013;28:848–854. - PubMed
-
- Anderson E.J., Lustig M.E., Boyle K.E., Woodlief T.L., Kane D.A., Lin C.T., Price J.W., 3rd, Kang L., Rabinovitch P.S., Szeto H.H. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 2009;119:573–581. - PMC - PubMed
-
- Auguet T., Terra X., Quintero Y., Martínez S., Manresa N., Porras J.A., Aguilar C., Orellana-Gavaldà J.M., Hernández M., Sabench F. Liver lipocalin 2 expression in severely obese women with non alcoholic fatty liver disease. Exp. Clin. Endocrinol. Diabetes. 2013;121:119–124. - PubMed
-
- Baffy G., Brunt E.M., Caldwell S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J. Hepatol. 2012;56:1384–1391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

