Novel Assay To Characterize Neutrophil Responses to Oral Biofilms
- PMID: 30455195
- PMCID: PMC6346121
- DOI: 10.1128/IAI.00790-18
Novel Assay To Characterize Neutrophil Responses to Oral Biofilms
Abstract
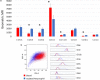
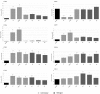
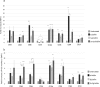
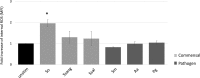
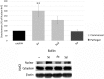
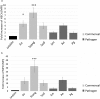
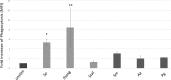
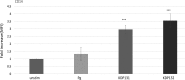
Neutrophils, the most numerous leukocytes, play an important role in maintaining oral health through interactions with oral microbial biofilms. Both neutrophil hyperactivity and the bacterial subversion of neutrophil responses can cause inflammation-mediated tissue damage like that seen in periodontal disease. We describe here an assay that assesses neutrophil activation responses to monospecies biofilm bacteria in vitro based on the surface expression of cluster of differentiation (CD) markers associated with various neutrophil functions. Most of what we know about neutrophil responses to bacteria is based on in vitro assays that use planktonic bacteria and isolated/preactivated neutrophils, which makes interpretation of the neutrophil responses to bacteria a challenge. An understanding of how neutrophils differentially interact with and respond to commensal and pathogenic oral bacteria is necessary in order to further understand the neutrophil's role in maintaining oral health and the pathogenesis of periodontal disease. In this study, a flow cytometry-based in vitro assay was developed to characterize neutrophil activation states based on CD marker expressions in response to oral monospecies bacterial biofilms. Using this approach, changes in CD marker expressions in response to specific prominent oral commensal and pathogenic bacteria were assayed. Several functional assays, including assays for phagocytosis, production of reactive oxygen species, activation of the transcription factor Nrf2, neutrophil extracellular trap formation, and myeloperoxidase release, were also performed to correlate neutrophil function with CD marker expression. Our results demonstrate that neutrophils display bacterial species-specific responses. This assay can be used to characterize how specific biofilms alter specific neutrophil pathways associated with their activation.
Keywords: CD markers; biofilm; commensal bacteria; immunology; microbiology; neutrophil; pathogens.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States.J Dent Res. 2016 Jul;95(8):931-8. doi: 10.1177/0022034516645564. Epub 2016 Jun 6. J Dent Res. 2016. PMID: 27270666
-
Mixed species biofilms of Fusobacterium necrophorum and Porphyromonas levii impair the oxidative response of bovine neutrophils in vitro.Anaerobe. 2017 Oct;47:157-164. doi: 10.1016/j.anaerobe.2017.05.008. Epub 2017 May 17. Anaerobe. 2017. PMID: 28526497
-
Tissue-specific murine neutrophil activation states in health and inflammation.J Leukoc Biol. 2021 Jul;110(1):187-195. doi: 10.1002/JLB.4AB1020-248RRR. Epub 2020 Nov 4. J Leukoc Biol. 2021. PMID: 33145850
-
The Role of NrF2 in the Regulation of Periodontal Health and Disease.J Dent Res. 2017 Aug;96(9):975-983. doi: 10.1177/0022034517715007. Epub 2017 Jun 15. J Dent Res. 2017. PMID: 28617616 Review.
-
Biofilms in Periprosthetic Orthopedic Infections Seen through the Eyes of Neutrophils: How Can We Help Neutrophils?Int J Mol Sci. 2023 Nov 23;24(23):16669. doi: 10.3390/ijms242316669. Int J Mol Sci. 2023. PMID: 38068991 Free PMC article. Review.
Cited by
-
The Crossroads of Periodontitis and Oral Squamous Cell Carcinoma: Immune Implications and Tumor Promoting Capacities.Front Oral Health. 2021 Jan 20;1:584705. doi: 10.3389/froh.2020.584705. eCollection 2020. Front Oral Health. 2021. PMID: 35047982 Free PMC article. Review.
-
Metronidazole enhances killing of Porphyromonas gingivalis by human PMNs.Front Oral Health. 2022 Aug 29;3:933997. doi: 10.3389/froh.2022.933997. eCollection 2022. Front Oral Health. 2022. PMID: 36105174 Free PMC article.
-
PAMPs and DAMPs as the Bridge Between Periodontitis and Atherosclerosis: The Potential Therapeutic Targets.Front Cell Dev Biol. 2022 Feb 25;10:856118. doi: 10.3389/fcell.2022.856118. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35281098 Free PMC article. Review.
-
The Association between Maternal Oral Inflammation and Neutrophil Phenotypes and Poly-Unsaturated Fatty Acids Composition in Human Milk: A Prospective Cohort Study.Cells. 2022 Dec 17;11(24):4110. doi: 10.3390/cells11244110. Cells. 2022. PMID: 36552874 Free PMC article.
-
Bidirectional effects of neutrophils on Streptococcus oralis biofilms in vitro.J Oral Microbiol. 2025 Jan 23;17(1):2453986. doi: 10.1080/20002297.2025.2453986. eCollection 2025. J Oral Microbiol. 2025. PMID: 39868359 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

