Effectiveness of an intervention to optimise the use of mirabegron for overactive bladder: a quasi-experimental study in primary care
- PMID: 30455222
- PMCID: PMC6255216
- DOI: 10.3399/bjgp18X699953
Effectiveness of an intervention to optimise the use of mirabegron for overactive bladder: a quasi-experimental study in primary care
Abstract
Background: Overactive bladder is a composite of lower urinary tract storage symptoms. Pharmacological treatment is widely employed despite markedly modest efficacy data, adverse effects, and costs for the health system.
Aim: To determine the 12-month efficacy of an intervention delivered by GPs on mirabegron revision and, if appropriate, discontinuation of treatment.
Design and setting: Multicentre, quasi-experimental study in Barcelona (Catalonia), Spain.
Method: Two groups composed of 17 intervention and 34 control practices were formed. The follow-up period was 12 months, from 1 January to 31 December 2017. A structured intervention was designed consisting of initiatives with GPs and urology/gynaecology specialists. The primary outcome was mirabegron use at 12 months.
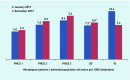
Results: Of the 1932 patients, a significant discontinuation in treatment was observed at 12 months' follow-up in the intervention group (IG) (n = 433 out of 762, 56.8%), in contrast with the control one (CG) (n = 484 out of 1170, 41.4%) (P<0.001). There was also a reduced incorporation of new treatments in the IG (n = 214 out of 762, 28.1%) compared with the CG (n = 595 out of 1170, 50.9%) (P<0.001). In relation to patients with treatment at the beginning and end of the period, there was a decrease of 219 (28.7%) patients in the IG and an increase of 111 (9.5%) in the CG (P<0.001).
Conclusion: The structured intervention showed optimisation in the use of mirabegron. When considering discontinuation it is necessary to provide clear data on the benefits and/or risks for patients and their caregivers, as such information is a precondition for shared decision making.
Keywords: costs; deprescribing; mirabegron; overactive bladder; patient-centred care; persistence (time to discontinuation).
© British Journal of General Practice 2018.
Figures




References
-
- Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50:1306–1314. - PubMed
-
- National Institute for Health and Care Excellence . Lower urinary tract symptoms in men: management CG97. London: NICE; 2010. [last updated May 2015] - PubMed
-
- National Institute for Health and Care Excellence . Urinary incontinence in women: management CG171. London: NICE; 2015.
-
- Burkhard FC, Bosch JLHR, Cruz F, et al. EAU Guidelines on urinary incontinence. European Association of Urology; 2017. http://uroweb.org/guideline/urinary-incontinence/?type=pocket-guidelines (accessed 30 Oct 2018)
-
- [No authors listed]. Mirabegron for overactive bladder syndrome. Drug Ther Bull. 2013;51(8):90–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
