Beyond Members of the Flaviviridae Family, Sofosbuvir Also Inhibits Chikungunya Virus Replication
- PMID: 30455237
- PMCID: PMC6355571
- DOI: 10.1128/AAC.01389-18
Beyond Members of the Flaviviridae Family, Sofosbuvir Also Inhibits Chikungunya Virus Replication
Abstract
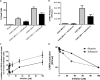
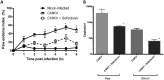
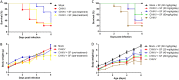
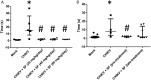
Chikungunya virus (CHIKV) causes a febrile disease associated with chronic arthralgia, which may progress to neurological impairment. Chikungunya fever (CF) is an ongoing public health problem in tropical and subtropical regions of the world, where control of the CHIKV vector, Aedes mosquitos, has failed. As there is no vaccine or specific treatment for CHIKV, patients receive only palliative care to alleviate pain and arthralgia. Thus, drug repurposing is necessary to identify antivirals against CHIKV. CHIKV RNA polymerase is similar to the orthologue enzyme of other positive-sense RNA viruses, such as members of the Flaviviridae family. Among the Flaviviridae, not only is hepatitis C virus RNA polymerase susceptible to sofosbuvir, a clinically approved nucleotide analogue, but so is dengue, Zika, and yellow fever virus replication. Here, we found that sofosbuvir was three times more selective in inhibiting CHIKV production in human hepatoma cells than ribavirin, a pan-antiviral drug. Although CHIKV replication in human induced pluripotent stem cell-derived astrocytes was less susceptible to sofosbuvir than were hepatoma cells, sofosbuvir nevertheless impaired virus production and cell death in a multiplicity of infection-dependent manner. Sofosbuvir also exhibited antiviral activity in vivo by preventing CHIKV-induced paw edema in adult mice at a dose of 20 mg/kg of body weight/day and prevented mortality in a neonate mouse model at 40- and 80-mg/kg/day doses. Our data demonstrate that a prototypic alphavirus, CHIKV, is also susceptible to sofosbuvir. As sofosbuvir is a clinically approved drug, our findings could pave the way to it becoming a therapeutic option against CF.
Keywords: antiviral; arthralgia; chikungunya; chikungunya virus; drug; sofosbuvir.
Copyright © 2019 American Society for Microbiology.
Figures

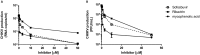

References
-
- Souza TMA, Azeredo EL, Badolato-Corrêa J, Damasco PV, Santos C, Petitinga-Paiva F, Nunes PCG, Barbosa LS, Cipitelli MC, Chouin-Carneiro T, Faria NRC, Nogueira RMR, de Bruycker-Nogueira F, dos Santos FB. 2017. First report of the East-Central South African genotype of chikungunya virus in Rio de Janeiro, Brazil. PLoS Curr 9:ecurrents.outbreaks.4200119978d62ccaa454599cd2735727. - PMC - PubMed
-
- Charlys da Costa A, Thézé J, Komninakis SCV, Sanz-Duro RL, Felinto MRL, Moura LCC, Barroso IM, Santos LEC, Nunes MA, Moura AA, Lourenço J, Deng X, Delwart EL, Guimarães MR, Pybus OG, Sabino EC, Faria NR. 2017. Spread of chikungunya virus East/Central/South African genotype in northeast Brazil. Emerg Infect Dis 23:1742–1744. doi: 10.3201/eid2310.170307. - DOI - PMC - PubMed
-
- Nunes MRT, Faria NR, de Vasconcelos JM, Golding N, Kraemer MU, de Oliveira LF, Azevedo RDSDS, Dea DS, Evp DS, da Silva SP, Carvalho VL, Coelho GE, Cruz ACR, Rodrigues SG, da Silva Gonçalves Vianez JL, Nunes BTD, Cardoso JF, Tesh RB, Hay SI, Pybus OG, da Costa Vasconcelos PF. 2015. Emergence and potential for spread of chikungunya virus in Brazil. BMC Med 13:102. doi: 10.1186/s12916-015-0348-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

