Artemisinin resistance phenotypes and K13 inheritance in a Plasmodium falciparum cross and Aotus model
- PMID: 30455312
- PMCID: PMC6298093
- DOI: 10.1073/pnas.1813386115
Artemisinin resistance phenotypes and K13 inheritance in a Plasmodium falciparum cross and Aotus model
Abstract
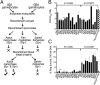
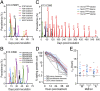
Concerns about malaria parasite resistance to treatment with artemisinin drugs (ARTs) have grown with findings of prolonged parasite clearance t1/2s (>5 h) and their association with mutations in Plasmodium falciparum Kelch-propeller protein K13. Here, we describe a P. falciparum laboratory cross of K13 C580Y mutant with C580 wild-type parasites to investigate ART response phenotypes in vitro and in vivo. After genotyping >400 isolated progeny, we evaluated 20 recombinants in vitro: IC50 measurements of dihydroartemisinin were at similar low nanomolar levels for C580Y- and C580-type progeny (mean ratio, 1.00; 95% CI, 0.62-1.61), whereas, in a ring-stage survival assay, the C580Y-type progeny had 19.6-fold (95% CI, 9.76-39.2) higher average counts. In splenectomized Aotus monkeys treated with three daily doses of i.v. artesunate, t1/2 calculations by three different methods yielded mean differences of 0.01 h (95% CI, -3.66 to 3.67), 0.80 h (95% CI, -0.92 to 2.53), and 2.07 h (95% CI, 0.77-3.36) between C580Y and C580 infections. Incidences of recrudescence were 57% in C580Y (4 of 7) versus 70% in C580 (7 of 10) infections (-13% difference; 95% CI, -58% to 35%). Allelic substitution of C580 in a C580Y-containing progeny clone (76H10) yielded a transformant (76H10C580Rev) that, in an infected monkey, recrudesced regularly 13 times over 500 d. Frequent recrudescences of ART-treated P. falciparum infections occur with or without K13 mutations and emphasize the need for improved partner drugs to effectively eliminate the parasites that persist through the ART component of combination therapy.
Keywords: artemisinin-based combination chemotherapy; genetic cross; malaria; parasite clearance time; recrudescence.
Conflict of interest statement
Conflict of interest statement: R.M.F. and D.E.G. are coauthors of a paper published in 2017. N.F.G., D.A.F., and D.E.G. are coauthors of papers published in 2016 and 2018. K.M., C.A.L., and D.E.G. are coauthors of a paper published in 2014. R.S.R. and S.R.M. are coauthors of papers published in 2015 and 2017.
Figures
References
-
- Li GQ, Arnold K, Guo XB, Jian HX, Fu LC. Randomised comparative study of mefloquine, qinghaosu, and pyrimethamine-sulfadoxine in patients with falciparum malaria. Lancet. 1984;2:1360–1361. - PubMed
-
- World Health Organization 1967 Chemotherapy of malaria: Report of a WHO scientific group (WHO, Geneva), Technical Report Series No. 375. Available at apps.who.int/iris/bitstream/handle/10665/40671/WHO_TRS_375.pdf. Accessed September 6, 2018.
-
- Looareesuwan S, et al. Randomised trial of artesunate and mefloquine alone and in sequence for acute uncomplicated falciparum malaria. Lancet. 1992;339:821–824. - PubMed
-
- Li GQ, Guo XB, Fu LC, Jian HX, Wang XH. Clinical trials of artemisinin and its derivatives in the treatment of malaria in China. Trans R Soc Trop Med Hyg. 1994;88(suppl 1):S5–S6. - PubMed
-
- Nguyen DS, et al. Treatment of malaria in Vietnam with oral artemisinin. Am J Trop Med Hyg. 1993;48:398–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

