Biological sex affects vaccine efficacy and protection against influenza in mice
- PMID: 30455317
- PMCID: PMC6298067
- DOI: 10.1073/pnas.1805268115
Biological sex affects vaccine efficacy and protection against influenza in mice
Abstract
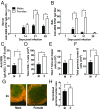
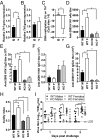
Biological sex affects adaptive immune responses, which could impact influenza infection and vaccine efficacy. Infection of mice with 2009 H1N1 induced antibody responses, CD4+ T cell and CD8+ T cell memory responses that were greater in females than males; both sexes, however, were equally protected against secondary challenge with an H1N1 drift variant virus. To test whether greater antibody in females is sufficient for protection against influenza, males and females were immunized with an inactivated H1N1 vaccine that induced predominantly antibody-mediated immunity. Following vaccination, females had greater antibody responses and protection against challenge with an H1N1 drift variant virus than males. Antibody derived from vaccinated females was better at protecting both naïve males and females than antibody from males, and this protection was associated with increased antibody specificity and avidity to the H1N1 virus. The expression of Tlr7 was greater in B cells from vaccinated females than males and was associated with reduced DNA methylation in the Tlr7 promoter region, higher neutralizing antibody, class switch recombination, and antibody avidity in females. Deletion of Tlr7 reduced sex differences in vaccine-induced antibody responses and protection following challenge and had a greater impact on responses in females than males. Taken together, these data illustrate that greater TLR7 activation and antibody production in females improves the efficacy of vaccination against influenza.
Keywords: CD8+ T cell memory; DNA methylation; antibody secreting cells; isotope switching; toll-like receptor 7.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–599. - PubMed
-
- Soema PC, Kompier R, Amorij JP, Kersten GF. Current and next generation influenza vaccines: Formulation and production strategies. Eur J Pharm Biopharm. 2015;94:251–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

