Single nucleotide polymorphisms alter kinase anchoring and the subcellular targeting of A-kinase anchoring proteins
- PMID: 30455320
- PMCID: PMC6298107
- DOI: 10.1073/pnas.1816614115
Single nucleotide polymorphisms alter kinase anchoring and the subcellular targeting of A-kinase anchoring proteins
Abstract
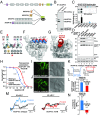
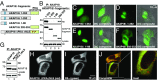
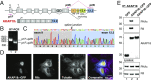
A-kinase anchoring proteins (AKAPs) shape second-messenger signaling responses by constraining protein kinase A (PKA) at precise intracellular locations. A defining feature of AKAPs is a helical region that binds to regulatory subunits (RII) of PKA. Mining patient-derived databases has identified 42 nonsynonymous SNPs in the PKA-anchoring helices of five AKAPs. Solid-phase RII binding assays confirmed that 21 of these amino acid substitutions disrupt PKA anchoring. The most deleterious side-chain modifications are situated toward C-termini of AKAP helices. More extensive analysis was conducted on a valine-to-methionine variant in the PKA-anchoring helix of AKAP18. Molecular modeling indicates that additional density provided by methionine at position 282 in the AKAP18γ isoform deflects the pitch of the helical anchoring surface outward by 6.6°. Fluorescence polarization measurements show that this subtle topological change reduces RII-binding affinity 8.8-fold and impairs cAMP responsive potentiation of L-type Ca2+ currents in situ. Live-cell imaging of AKAP18γ V282M-GFP adducts led to the unexpected discovery that loss of PKA anchoring promotes nuclear accumulation of this polymorphic variant. Targeting proceeds via a mechanism whereby association with the PKA holoenzyme masks a polybasic nuclear localization signal on the anchoring protein. This led to the discovery of AKAP18ε: an exclusively nuclear isoform that lacks a PKA-anchoring helix. Enzyme-mediated proximity-proteomics reveal that compartment-selective variants of AKAP18 associate with distinct binding partners. Thus, naturally occurring PKA-anchoring-defective AKAP variants not only perturb dissemination of local second-messenger responses, but also may influence the intracellular distribution of certain AKAP18 isoforms.
Keywords: AKAPs; PKA; kinase anchoring; nucleus; proximity labeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Investigating PKA-RII specificity using analogs of the PKA:AKAP peptide inhibitor STAD-2.Bioorg Med Chem. 2018 Mar 15;26(6):1174-1178. doi: 10.1016/j.bmc.2018.02.001. Epub 2018 Feb 12. Bioorg Med Chem. 2018. PMID: 29449124 Free PMC article.
-
Analysis of A-kinase anchoring protein (AKAP) interaction with protein kinase A (PKA) regulatory subunits: PKA isoform specificity in AKAP binding.J Mol Biol. 2000 Apr 28;298(2):329-39. doi: 10.1006/jmbi.2000.3662. J Mol Biol. 2000. PMID: 10764601
-
A systematic evaluation of protein kinase A-A-kinase anchoring protein interaction motifs.Biochemistry. 2015 Jan 13;54(1):11-21. doi: 10.1021/bi500721a. Epub 2014 Sep 10. Biochemistry. 2015. PMID: 25097019
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
-
Compartmentalization role of A-kinase anchoring proteins (AKAPs) in mediating protein kinase A (PKA) signaling and cardiomyocyte hypertrophy.Int J Mol Sci. 2014 Dec 24;16(1):218-29. doi: 10.3390/ijms16010218. Int J Mol Sci. 2014. PMID: 25547489 Free PMC article. Review.
Cited by
-
From Affinity to Proximity Techniques to Investigate Protein Complexes in Plants.Int J Mol Sci. 2021 Jul 1;22(13):7101. doi: 10.3390/ijms22137101. Int J Mol Sci. 2021. PMID: 34281155 Free PMC article. Review.
-
The Role of the Popeye Domain Containing Gene Family in Organ Homeostasis.Cells. 2019 Dec 7;8(12):1594. doi: 10.3390/cells8121594. Cells. 2019. PMID: 31817925 Free PMC article. Review.
-
Recruitment of BAG2 to DNAJ-PKAc scaffolds promotes cell survival and resistance to drug-induced apoptosis in fibrolamellar carcinoma.Cell Rep. 2024 Feb 27;43(2):113678. doi: 10.1016/j.celrep.2024.113678. Epub 2024 Jan 17. Cell Rep. 2024. PMID: 38236773 Free PMC article.
-
Chemical biology approaches to resolve the subcellular GPCR signaling landscape.Nat Chem Biol. 2025 Aug;21(8):1148-1159. doi: 10.1038/s41589-025-01928-x. Epub 2025 Jun 2. Nat Chem Biol. 2025. PMID: 40456961 Free PMC article. Review.
-
CG-NAP/Kinase Interactions Fine-Tune T Cell Functions.Front Immunol. 2019 Nov 12;10:2642. doi: 10.3389/fimmu.2019.02642. eCollection 2019. Front Immunol. 2019. PMID: 31781123 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

