A cell separation checkpoint that enforces the proper order of late cytokinetic events
- PMID: 30455324
- PMCID: PMC6314563
- DOI: 10.1083/jcb.201805100
A cell separation checkpoint that enforces the proper order of late cytokinetic events
Abstract
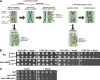
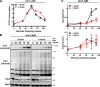
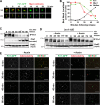
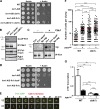
Eukaryotic cell division requires dependency relationships in which late processes commence only after early ones are appropriately completed. We have discovered a system that blocks late events of cytokinesis until early ones are successfully accomplished. In budding yeast, cytokinetic actomyosin ring contraction and membrane ingression are coupled with deposition of an extracellular septum that is selectively degraded in its primary septum immediately after its completion by secreted enzymes. We find this secretion event is linked to septum completion and forestalled when the process is slowed. Delay of septum degradation requires Fir1, an intrinsically disordered protein localized to the cytokinesis site that is degraded upon septum completion but stabilized when septation is aberrant. Fir1 protects cytokinesis in part by inhibiting a separation-specific exocytosis function of the NDR/LATS kinase Cbk1, a key component of "hippo" signaling that induces mother-daughter separation. We term this system enforcement of cytokinesis order, a checkpoint ensuring proper temporal sequence of mechanistically incompatible processes of cytokinesis.
© 2018 Brace et al.
Figures



Similar articles
-
Mitotic exit control of the Saccharomyces cerevisiae Ndr/LATS kinase Cbk1 regulates daughter cell separation after cytokinesis.Mol Cell Biol. 2011 Feb;31(4):721-35. doi: 10.1128/MCB.00403-10. Epub 2010 Dec 6. Mol Cell Biol. 2011. PMID: 21135117 Free PMC article.
-
Aim44p regulates phosphorylation of Hof1p to promote contractile ring closure during cytokinesis in budding yeast.Mol Biol Cell. 2014 Mar;25(6):753-62. doi: 10.1091/mbc.E13-06-0317. Epub 2014 Jan 22. Mol Biol Cell. 2014. PMID: 24451263 Free PMC article.
-
Lre1 directly inhibits the NDR/Lats kinase Cbk1 at the cell division site in a phosphorylation-dependent manner.Curr Biol. 2013 Sep 23;23(18):1736-45. doi: 10.1016/j.cub.2013.07.032. Epub 2013 Aug 15. Curr Biol. 2013. PMID: 23954433
-
Mechanics and regulation of cytokinesis in budding yeast.Semin Cell Dev Biol. 2017 Jun;66:107-118. doi: 10.1016/j.semcdb.2016.12.010. Epub 2016 Dec 27. Semin Cell Dev Biol. 2017. PMID: 28034796 Free PMC article. Review.
-
Cell separation and the maintenance of cell integrity during cytokinesis in yeast: the assembly of a septum.Yeast. 2010 Aug;27(8):521-30. doi: 10.1002/yea.1779. Yeast. 2010. PMID: 20641019 Review.
Cited by
-
TOR complex 1 negatively regulates NDR kinase Cbk1 to control cell separation in budding yeast.PLoS Biol. 2023 Aug 30;21(8):e3002263. doi: 10.1371/journal.pbio.3002263. eCollection 2023 Aug. PLoS Biol. 2023. PMID: 37647291 Free PMC article.
-
The Mitogen-Activated Protein Kinase Slt2 Promotes Asymmetric Cell Cycle Arrest and Reduces TORC1-Sch9 Signaling in Yeast Lacking the Protein Phosphatase Ptc1.Microbiol Spectr. 2023 Jun 15;11(3):e0524922. doi: 10.1128/spectrum.05249-22. Epub 2023 Apr 12. Microbiol Spectr. 2023. PMID: 37042757 Free PMC article.
-
Effects of 5'-3' Exonuclease Xrn1 on Cell Size, Proliferation and Division, and mRNA Levels of Periodic Genes in Cryptococcus neoformans.Genes (Basel). 2020 Apr 16;11(4):430. doi: 10.3390/genes11040430. Genes (Basel). 2020. PMID: 32316250 Free PMC article.
-
Cytokinesis in Eukaryotic Cells: The Furrow Complexity at a Glance.Cells. 2020 Jan 22;9(2):271. doi: 10.3390/cells9020271. Cells. 2020. PMID: 31979090 Free PMC article. Review.
-
A time-resolved interaction analysis of Bem1 reconstructs the flow of Cdc42 during polar growth.Life Sci Alliance. 2020 Jul 31;3(9):e202000813. doi: 10.26508/lsa.202000813. Print 2020 Sep. Life Sci Alliance. 2020. PMID: 32737079 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

