Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis-induced lung injury and inflammation
- PMID: 30455348
- PMCID: PMC6333887
- DOI: 10.1074/jbc.RA118.004638
Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis-induced lung injury and inflammation
Abstract
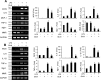
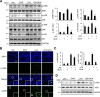
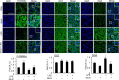
The aim of the current study was to investigate the effects of glucosamine (GlcN) on septic lethality and sepsis-induced inflammation using animal models of mice and zebrafish. GlcN pretreatment improved survival in the cecal ligation and puncture (CLP)-induced sepsis mouse model and attenuated lipopolysaccharide (LPS)-induced septic lung injury and systemic inflammation. GlcN suppressed LPS-induced M1-specific but not M2-specific gene expression. Furthermore, increased expressions of inflammatory genes in visceral tissue of LPS-injected zebrafish were suppressed by GlcN. GlcN suppressed LPS-induced activation of mitogen-activated protein kinase (MAPK) and NF-κB in lung tissue. LPS triggered a reduction in O-GlcNAc levels in nucleocytoplasmic proteins of lung, liver, and spleen after 1 day, which returned to normal levels at day 3. GlcN inhibited LPS-induced O-GlcNAc down-regulation in mouse lung and visceral tissue of zebrafish. Furthermore, the O-GlcNAcase (OGA) level was increased by LPS, which were suppressed by GlcN in mouse and zebrafish. OGA inhibitors suppressed LPS-induced expression of inflammatory genes in RAW264.7 cells and the visceral tissue of zebrafish. Stable knockdown of Oga via short hairpin RNA led to increased inducible nitric oxide synthase (iNOS) expression in response to LPS with or without GlcN in RAW264.7 cells. Overall, our results demonstrate a protective effect of GlcN on sepsis potentially through modulation of O-GlcNAcylation of nucleocytoplasmic proteins.
Keywords: O-GlcNAcylation; glucose metabolism; inflammation; lung injury; sepsis.
© 2019 Hwang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures







References
-
- Fleischmann C., Scherag A., Adhikari N. K., Hartog C. S., Tsaganos T., Schlattmann P., Angus D. C., Reinhart K., and International Forum of Acute Care Trialists (2016) Assessment of global incidence and mortality of hospital-treated sepsis: current estimates and limitations. Am. J. Respir. Crit. Care Med. 193, 259–272 10.1164/rccm.201504-0781OC - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

