Leucine-Rich Alpha-2-Glycoprotein1 Gene Interferes with Regulation of Apoptosis in Leukemia KASUMI-1 Cells
- PMID: 30455412
- PMCID: PMC6256841
- DOI: 10.12659/MSM.911249
Leucine-Rich Alpha-2-Glycoprotein1 Gene Interferes with Regulation of Apoptosis in Leukemia KASUMI-1 Cells
Abstract
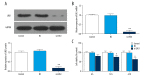
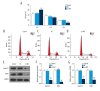
BACKGROUND Leukemia cells have strong proliferation and anti-apoptosis capabilities. The purpose of this study was to investigate the effect of silencing the leucine-rich alpha-2-glycoprotein1 (LRG1) gene, which was found to regulate tumor proliferation and apoptosis in acute myeloid leukemia (AML) cell lines. MATERIAL AND METHODS Plasmid interference technique was used to silence the LRG1 gene in the KASUMI-1 cell line. The cell counting kit-8 (CCK-8) assay was used to test the effect of transduction on cell viability. Cell cycle and apoptosis were detected by flow cytometry. Western blot and quantitative real-time polymerase chain reaction (RT-qPCR) were applied to detect the expression levels of proteins and mRNA, respectively. RESULTS KASUMI-1 cells with the CD34⁺CD38⁻ phenotype were sorted by flow cytometry. After transfection of the siLRG1 plasmid, the level of LRG1 expression was downregulated and cell viability was reduced. Silencing of LRG1 gene blocked KASUMI-1 cells in G0/G1 phase and promoted apoptosis. Further experiments found that LRG1 gene silencing significantly downregulated cell cycle-associated proteins and anti-apoptotic proteins, while upregulating pro-apoptotic proteins. Downregulation of LRG1 gene expression also inhibits signal transduction of the JAK-STAT pathway. CONCLUSIONS LRG1 gene silencing regulates the expression of cyclin and apoptosis-related proteins to reduce cell viability and promote apoptosis, probably through inhibition of the JAK-STAT pathway.
Figures


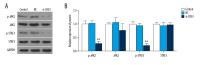
Similar articles
-
Research Progress on Leucine-Rich Alpha-2 Glycoprotein 1: A Review.Front Pharmacol. 2022 Jan 5;12:809225. doi: 10.3389/fphar.2021.809225. eCollection 2021. Front Pharmacol. 2022. PMID: 35095520 Free PMC article. Review.
-
Epigenetic Silencing of Eyes Absent 4 Gene by Acute Myeloid Leukemia 1-Eight-twenty-one Oncoprotein Contributes to Leukemogenesis in t(8;21) Acute Myeloid Leukemia.Chin Med J (Engl). 2016 Jun 5;129(11):1355-62. doi: 10.4103/0366-6999.182838. Chin Med J (Engl). 2016. PMID: 27231175 Free PMC article.
-
Stable knockdown of LRG1 by RNA interference inhibits growth and promotes apoptosis of glioblastoma cells in vitro and in vivo.Tumour Biol. 2015 Jun;36(6):4271-8. doi: 10.1007/s13277-015-3065-3. Epub 2015 Jan 15. Tumour Biol. 2015. PMID: 25589464
-
MiR-212-5p regulates the proliferation and apoptosis of AML cells through targeting FZD5.Eur Rev Med Pharmacol Sci. 2018 Dec;22(23):8415-8422. doi: 10.26355/eurrev_201812_16540. Eur Rev Med Pharmacol Sci. 2018. PMID: 30556883
-
LRG1 in cancer: mechanisms, potential therapeutic target, and use in clinical diagnosis.Mol Biol Rep. 2025 Jun 10;52(1):577. doi: 10.1007/s11033-025-10669-y. Mol Biol Rep. 2025. PMID: 40493117 Review.
Cited by
-
Research Progress on Leucine-Rich Alpha-2 Glycoprotein 1: A Review.Front Pharmacol. 2022 Jan 5;12:809225. doi: 10.3389/fphar.2021.809225. eCollection 2021. Front Pharmacol. 2022. PMID: 35095520 Free PMC article. Review.
-
High serum levels of leucine-rich α-2 glycoprotein 1 (LRG-1) are associated with poor survival in patients with early breast cancer.Arch Gynecol Obstet. 2024 Jun;309(6):2789-2798. doi: 10.1007/s00404-024-07434-0. Epub 2024 Feb 28. Arch Gynecol Obstet. 2024. PMID: 38413424 Free PMC article.
-
LRG1: an emerging player in disease pathogenesis.J Biomed Sci. 2022 Jan 21;29(1):6. doi: 10.1186/s12929-022-00790-6. J Biomed Sci. 2022. PMID: 35062948 Free PMC article. Review.
-
Paradoxical Roles of Leucine-Rich α2-Glycoprotein-1 in Cell Death and Survival Modulated by Transforming Growth Factor-Beta 1 and Cytochrome c.Front Cell Dev Biol. 2021 Oct 8;9:744908. doi: 10.3389/fcell.2021.744908. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34692699 Free PMC article.
-
miR-18a Inhibitor Suppresses Leukemia Cell Proliferation by Upregulation of PTEN Expression.Med Sci Monit. 2020 Mar 8;26:e921288. doi: 10.12659/MSM.921288. Med Sci Monit. 2020. PMID: 32146479 Free PMC article.
References
-
- Ofran Y, Rowe JM. Acute myeloid leukemia in adolescents and young adults: Challenging aspects. Acta Haematol. 2014;132(3–4):292–97. - PubMed
-
- Haupt H, Baudner S. [Isolation and characterization of an unknown, leucine-rich 3.1-S-alpha2-glycoprotein from human serum (author’s transl)]. Hoppe Seylers Z Physiol Chem. 1977;358(6):639–46. [in German] - PubMed
-
- Serada S, Fujimoto M, Terabe F, et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis. 2012;18(11):2169–79. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

