DEL-1 promotes macrophage efferocytosis and clearance of inflammation
- PMID: 30455459
- PMCID: PMC6291356
- DOI: 10.1038/s41590-018-0249-1
DEL-1 promotes macrophage efferocytosis and clearance of inflammation
Abstract
Resolution of inflammation is essential for tissue homeostasis and represents a promising approach to inflammatory disorders. Here we found that developmental endothelial locus-1 (DEL-1), a secreted protein that inhibits leukocyte-endothelial adhesion and inflammation initiation, also functions as a non-redundant downstream effector in inflammation clearance. In human and mouse periodontitis, waning of inflammation was correlated with DEL-1 upregulation, whereas resolution of experimental periodontitis failed in DEL-1 deficiency. This concept was mechanistically substantiated in acute monosodium-urate-crystal-induced inflammation, where the pro-resolution function of DEL-1 was attributed to effective apoptotic neutrophil clearance (efferocytosis). DEL-1-mediated efferocytosis induced liver X receptor-dependent macrophage reprogramming to a pro-resolving phenotype and was required for optimal production of at least certain specific pro-resolving mediators. Experiments in transgenic mice with cell-specific overexpression of DEL-1 linked its anti-leukocyte-recruitment action to endothelial cell-derived DEL-1 and its efferocytic/pro-resolving action to macrophage-derived DEL-1. Thus, the compartmentalized expression of DEL-1 facilitates distinct homeostatic functions in an appropriate context that can be harnessed therapeutically.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
All authors declare that they have no competing interests.
Figures
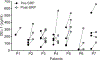
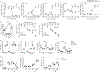



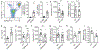
Comment in
-
DELineating resolution of inflammation.Nat Immunol. 2019 Jan;20(1):2-3. doi: 10.1038/s41590-018-0278-9. Nat Immunol. 2019. PMID: 30538337 No abstract available.
References
-
- Fullerton JN & Gilroy DW Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov 15, 551–567 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

