Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery
- PMID: 30455461
- PMCID: PMC6242930
- DOI: 10.1038/s41419-018-1190-9
Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery
Abstract
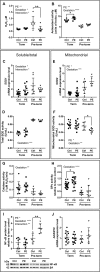
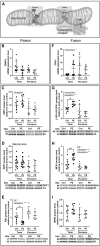
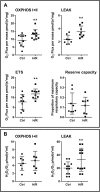
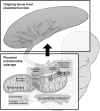
Preeclampsia is a devastating pregnancy disorder. Severity varies widely, and while severe preeclampsia often requires pre-term delivery, women with mild preeclampsia may reach term with minor interventions. The mechanisms that mediate disease severity are poorly understood, but may include adaptive processes by the placenta. We aimed to establish whether in pregnancies that reached term and those that delivered pre-term, the placental response to preeclampsia was intrinsically different, and explore potential adaptive mechanisms. Hydrogen peroxide production and antioxidant activity were increased in term preeclamptic placentae, whereas pre-term preeclamptic placentae had reduced hydrogen peroxide production and reduced function of the antioxidant system superoxide dismutase compared to control placentae. Markers of mitochondrial fission/fusion, apoptosis and the expression level of mitochondrial complexes were differentially disrupted in term compared to pre-term preeclamptic placentae. Mitochondrial respiration and content were increased in term preeclamptic placentae, but mitochondria had a lower respiratory reserve capacity. Mitochondrial respiration and hydrogen peroxide production were increased in healthy term placentae after in vitro hypoxia/reoxygenation. Placentae from preeclamptic pregnancies that reached term showed multiple adaptions that were not present in pre-term preeclamptic placentae. Increased antioxidant activity, and expression of markers of mitochondrial fusion and apoptotic suppression, may relate to salvaging damaged mitochondria. Increased mitochondrial respiration may allow ongoing tissue function even with reduced respiratory efficiency in term preeclamptic pregnancies. Response after in vitro hypoxia/reoxygenation suggests that disruption of oxygen supply is key to placental mitochondrial adaptations. Reactive oxygen species signalling in term preeclamptic placentae may be at a level to trigger compensatory antioxidant and mitochondrial responses, allowing tissue level maintenance of function when there is organelle level dysfunction.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Placental Mitochondrial Abnormalities in Preeclampsia.Reprod Sci. 2021 Aug;28(8):2186-2199. doi: 10.1007/s43032-021-00464-y. Epub 2021 Feb 1. Reprod Sci. 2021. PMID: 33523425 Free PMC article.
-
Reduced expression of survivin, the inhibitor of apoptosis protein correlates with severity of preeclampsia.Placenta. 2012 Jan;33(1):47-51. doi: 10.1016/j.placenta.2011.10.008. Epub 2011 Oct 26. Placenta. 2012. PMID: 22033156
-
Sulforaphane improves syncytiotrophoblast mitochondrial function after in vitro hypoxic and superoxide injury.Placenta. 2020 Jul;96:44-54. doi: 10.1016/j.placenta.2020.05.005. Epub 2020 May 19. Placenta. 2020. PMID: 32560857
-
Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy.Clin Exp Pharmacol Physiol. 2020 Jan;47(1):176-184. doi: 10.1111/1440-1681.13172. Epub 2019 Sep 15. Clin Exp Pharmacol Physiol. 2020. PMID: 31469913 Review.
-
Morphological changes of placental syncytium and their implications for the pathogenesis of preeclampsia.Cell Mol Life Sci. 2016 Jan;73(2):365-76. doi: 10.1007/s00018-015-2069-x. Epub 2015 Oct 26. Cell Mol Life Sci. 2016. PMID: 26496726 Free PMC article. Review.
Cited by
-
The trichloroethylene metabolite S-(1,2-dichlorovinyl)-L-cysteine induces progressive mitochondrial dysfunction in HTR-8/SVneo trophoblasts.Toxicology. 2019 Nov 1;427:152283. doi: 10.1016/j.tox.2019.152283. Epub 2019 Aug 30. Toxicology. 2019. PMID: 31476333 Free PMC article.
-
Mitochondrial Dysfunction in the Pathogenesis of Preeclampsia.Curr Hypertens Rep. 2022 Jun;24(6):157-172. doi: 10.1007/s11906-022-01184-7. Epub 2022 Mar 7. Curr Hypertens Rep. 2022. PMID: 35254588 Free PMC article. Review.
-
Pathophysiology of Preeclampsia and L-Arginine/L-Citrulline Supplementation as a Potential Strategy to Improve Birth Outcomes.Adv Exp Med Biol. 2023;1428:127-148. doi: 10.1007/978-3-031-32554-0_6. Adv Exp Med Biol. 2023. PMID: 37466772 Review.
-
The Nitrate-Nitrite-Nitric Oxide Pathway: Potential Role in Mitigating Oxidative Stress in Hypertensive Disorders of Pregnancy.Nutrients. 2024 May 14;16(10):1475. doi: 10.3390/nu16101475. Nutrients. 2024. PMID: 38794713 Free PMC article. Review.
-
Cryopreserved placental biopsies maintain mitochondrial activity for high-resolution respirometry.Mol Med. 2023 Apr 3;29(1):45. doi: 10.1186/s10020-023-00645-2. Mol Med. 2023. PMID: 37013473 Free PMC article.
References
-
- Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best practice & research. Clin. Obstet. Gynaecol. 2011;25:391–403. - PubMed
-
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 122, 1122–31. 10.1097/01.AOG.0000437382.03963.88. (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

