Lentiviral transduction of mammalian cells for fast, scalable and high-level production of soluble and membrane proteins
- PMID: 30455477
- PMCID: PMC6364805
- DOI: 10.1038/s41596-018-0075-9
Lentiviral transduction of mammalian cells for fast, scalable and high-level production of soluble and membrane proteins
Abstract
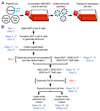
Structural, biochemical and biophysical studies of eukaryotic soluble and membrane proteins require their production in milligram quantities. Although large-scale protein expression strategies based on transient or stable transfection of mammalian cells are well established, they are associated with high consumable costs, limited transfection efficiency or long and tedious selection of clonal cell lines. Lentiviral transduction is an efficient method for the delivery of transgenes to mammalian cells and unifies the ease of use and speed of transient transfection with the robust expression of stable cell lines. In this protocol, we describe the design and step-by-step application of a lentiviral plasmid suite, termed pHR-CMV-TetO2, for the constitutive or inducible large-scale production of soluble and membrane proteins in HEK293 cell lines. Optional features include bicistronic co-expression of fluorescent marker proteins for enrichment of co-transduced cells using cell sorting and of biotin ligase for in vivo biotinylation. We demonstrate the efficacy of the method for a set of soluble proteins and for the G-protein-coupled receptor (GPCR) Smoothened (SMO). We further compare this method with baculovirus transduction of mammalian cells (BacMam), using the type-A γ-aminobutyric acid receptor (GABAAR) β3 homopentamer as a test case. The protocols described here are optimized for simplicity, speed and affordability; lead to a stable polyclonal cell line and milligram-scale amounts of protein in 3-4 weeks; and routinely achieve an approximately three- to tenfold improvement in protein production yield per cell as compared to transient transduction or transfection.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta crystallographica. Section D, Biological crystallography. 2006;62:1243–1250. - PubMed
-
- Naldini L, Trono D, Verma IM. Lentiviral vectors, two decades later. Science. 2016;353:1101–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/L009609/1/MRC_/Medical Research Council/United Kingdom
- 17721/CRUK_/Cancer Research UK/United Kingdom
- MC_UP_1201/15/MRC_/Medical Research Council/United Kingdom
- MR/M000141/1/MRC_/Medical Research Council/United Kingdom
- 26752/CRUK_/Cancer Research UK/United Kingdom
- 14414/CRUK_/Cancer Research UK/United Kingdom
- MR/N00065X/1/MRC_/Medical Research Council/United Kingdom
- MC_EX_MR/L009609/2/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- G1100525/MRC_/Medical Research Council/United Kingdom
- MR/L017776/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

