Cloning, purification, and characterization of an organic solvent-tolerant chitinase, MtCh509, from Microbulbifer thermotolerans DAU221
- PMID: 30455732
- PMCID: PMC6222997
- DOI: 10.1186/s13068-018-1299-1
Cloning, purification, and characterization of an organic solvent-tolerant chitinase, MtCh509, from Microbulbifer thermotolerans DAU221
Abstract
Background: The ability to use organic solvents in enzyme reactions offers a number of industrially useful advantages. However, most enzymes are almost completely inactive in the presence of organic solvents. Thus, organic solvent-tolerant enzymes have potential applications in industrial processes.
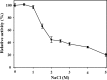
Results: A chitinase gene from Microbulbifer thermotolerans DAU221 (mtch509) was cloned and expressed in Escherichia coli BL21 (DE3). The molecular weight of the expressed MtCh509 protein was approximately 60 kDa, and it was purified by His-tag affinity chromatography. Enzymatic assays showed that the optimum temperature for MtCh509 chitinase activity was 55 °C, and the enzyme was stable for 2 h at up to 50 °C. The optimum pH for MtCh509 activity was in the sub-acidic range, at pH 4.6 and 5.0. The activity of MtCh509 was maintained in presence of 1 M salt, gradually decreasing at higher concentrations, with residual activity (20%) detected after incubation in 5 M salt. Some organic solvents (benzene, DMSO, hexane, isoamyl alcohol, isopropyl alcohol, and toluene; 10-20%, v/v) increased the reactivity of MtCh509 relative to the aqueous system. When using NAG3, as a substrate, MtCh509 produced NAG2 as the major product, as well as NAG4, demonstrating that MtCh509 has transglycosylation activity. The K m and V max values for MtCh509 using colloidal chitin as a substrate were 9.275 mg/mL and 20.4 U/mg, respectively. Thus, MtCh509 could be used in extreme industrial conditions.
Conclusion: The results of the hydrolysate analysis and the observed increase in enzyme activity in the presence of organic solvents show that MtCh509 has industrially attractive advantages. This is the first report on an organic solvent-tolerant and transglycosylating chitinase from Microbulbifer species.
Keywords: DAU221; Microbulbifer thermotolerans; Organic solvent-tolerant chitinase; Transglycosylation.
Figures





References
-
- Kim TI, Ki KS, Lim DH, Vijayakumar M, Park SM, Choi SH, Kim KY, Im SK, Park BY. Novel Acinetobacter parvus HANDI 309 microbial biomass for the production of N-acetyl-β-d-glucosamine (GlcNAc) using swollen chitin substrate in submerged fermentation. Biotechnol Biofuels. 2017;10:59. doi: 10.1186/s13068-017-0740-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

