Copper Chaperone Atox1 Interacts with Cell Cycle Proteins
- PMID: 30455854
- PMCID: PMC6231052
- DOI: 10.1016/j.csbj.2018.10.018
Copper Chaperone Atox1 Interacts with Cell Cycle Proteins
Abstract
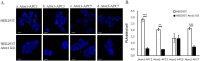
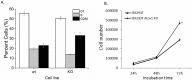
The anaphase-promoting complex (APC) is involved in several processes in the cell cycle, most prominently it facilitates the separation of the sister chromatids during mitosis, before cell division. Because of the key role in the cell cycle, APC is suggested as a putative target for anticancer agents. We here show that the copper chaperone Atox1, known for shuttling copper in the cytoplasm from Ctr1 to ATP7A/B in the secretory pathway, interacts with several APC subunits. Atox1 interactions with APC subunits were discovered by mass spectrometry of co-immunoprecipitated samples and further confirmed using proximity ligation assays in HEK293T cells. Upon comparing wild-type cells with those in which the Atox1 gene had been knocked out, we found that in the absence of Atox1 protein, cells have prolonged G2/M phases and a slower proliferation rate. Thus, in addition to copper transport for loading of copper-dependent enzymes, Atox1 may modulate the cell cycle by interacting with APC subunits.
Figures
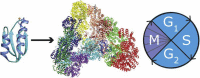

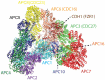
References
-
- Huffman D.L., O'Halloran T.V. Function, structure, and mechanism of intracellular copper trafficking proteins. Annu Rev Biochem. 2001;70:677–701. - PubMed
-
- Puig S., Thiele D.J. Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol. 2002;6:171–180. - PubMed
-
- Harris E.D. Basic and clinical aspects of copper. Crit Rev Clin Lab Sci. 2003;40:547–586. - PubMed
-
- Valko M., Morris H., Cronin M.T. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. - PubMed
-
- O'Halloran T.V., Culotta V.C. Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem. 2000;275:25057–25060. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
