Pharmacoinformatic Approach to Explore the Antidote Potential of Phytochemicals on Bungarotoxin from Indian Krait, Bungarus caeruleus
- PMID: 30455855
- PMCID: PMC6231056
- DOI: 10.1016/j.csbj.2018.10.005
Pharmacoinformatic Approach to Explore the Antidote Potential of Phytochemicals on Bungarotoxin from Indian Krait, Bungarus caeruleus
Abstract
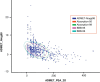
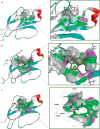
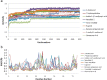
Venomous reptiles especially serpents are well known for their adverse effects after accidental conflicts with humans. Upon biting humans these serpents transmit arrays of detrimental toxins with diverse physiological activities that may either lead to minor symptoms such as dermatitis and allergic response or highly severe symptoms such as blood coagulation, disseminated intravascular coagulation, tissue injury, and hemorrhage. Other complications like respiratory arrest and necrosis may also occur. Bungarotoxins are a group of closely related neurotoxic proteins derived from the venom of kraits (Bungarus caeruleus) one of the six most poisonous snakes in India whose bite causes respiratory paralysis and mortality without showing any local symptoms. In the current study, by employing various pharmacoinformatic approaches, we have explored the antidote properties of 849 bioactive phytochemicals from 82 medicinal plants which have already shown antidote properties against various venomous toxins. These herbal compounds were taken and pharmacoinformatic approaches such as ADMET, docking and molecular dynamics were employed. The three-dimensional modelling approach provides structural insights on the interaction between bungarotoxin and phytochemicals. In silico simulations proved to be an effective analytical tools to investigate the toxin-ligand interaction, correlating with the affinity of binding. By analyzing the results from the present study, we proposed nine bioactive phytochemical compounds which are, 2-dodecanol, 7-hydroxycadalene, indole-3-(4'-oxo)butyric acid, nerolidol-2, trans-nerolidol, eugenol, benzene propanoic acid, 2-methyl-1-undecanol, germacren-4-ol can be used as antidotes for bungarotoxin.
Keywords: Bungarotoxin; Drug design; Molecular docking; Molecular dynamics; Pharmacokinetic profiling; Toxins.
Figures







References
-
- Mackessy S.P. CRC Press; 2016. Handbook of venoms and toxins of reptiles.
-
- Chang C.C. Looking back on the discovery of alpha-bungarotoxin. J Biomed Sci. 1999;6(6):368–375. - PubMed
LinkOut - more resources
Full Text Sources
