Diffusion-weighted imaging (DWI) in lymph node staging for prostate cancer
- PMID: 30456184
- PMCID: PMC6212625
- DOI: 10.21037/tau.2018.08.04
Diffusion-weighted imaging (DWI) in lymph node staging for prostate cancer
Abstract
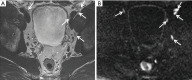
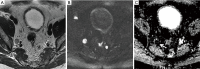
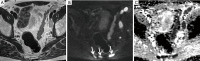
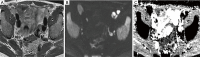
In patients with prostate cancer, the presence of lymph node (LN) metastases is a critical prognostic factor and is essential for treatment planning. Conventional cross-sectional imaging performs poorly for nodal staging as both computed tomography (CT) and magnetic resonance imaging (MRI) are mainly dependent on size and basic morphological criteria. Therefore, extended pelvic LN dissection (ePLND) remains the gold standard for LN staging, however, it is an invasive procedure with its own drawbacks, thus creating a need for accurate preoperative imaging test. Incorporating functional MRI by using diffusion-weighted MRI has proven superior to conventional MRI protocol by means of both qualitative and quantitative assessment. Currently, the increased diagnostic performance remains insufficient to replace ePLND and the future role of DWI may be through combination with MR lymphangiography or with novel positron emission tomography (PET) tracers. In this article, the current state of data supporting DWI in LN staging of patients with prostate cancer is discussed.
Keywords: Diffusion-weighted imaging (DWI); lymph nodes (LNs); magnetic resonance imaging (MRI); prostate cancer (PCa); staging.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
