Cellular Phenotype Plasticity in Cancer Dormancy and Metastasis
- PMID: 30456206
- PMCID: PMC6230580
- DOI: 10.3389/fonc.2018.00505
Cellular Phenotype Plasticity in Cancer Dormancy and Metastasis
Abstract
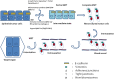
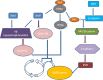
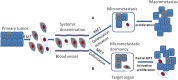
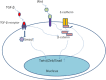
Cancer dormancy is a period of cancer progression in which residual tumor cells exist, but clinically remain asymptomatic for a long time, as well as resistant to conventional chemo- and radiotherapies. Cellular phenotype plasticity represents that cellular phenotype could convert between epithelial cells and cells with mesenchymal traits. Recently, this process has been shown to closely associate with tumor cell proliferation, cancer dormancy and metastasis. In this review, we have described different scenarios of how the transition from epithelial to mesenchymal morphology (EMT) and backwards (MET) are connected with the initiation of dormancy and reactivation of proliferation. These processes are fundamental for cancer cells to invade tissues and metastasize. Recognizing the mechanisms underlying the cellular phenotype plasticity as well as dormancy and targeting them is likely to increase the efficiency of traditional tumor treatment inhibiting tumor metastasis.
Keywords: EMT; MET; cancer cell dormancy; cancer metastasis; cellular phenotype plasticity.
Figures
Similar articles
-
PRRX1 Regulates Cellular Phenotype Plasticity and Dormancy of Head and Neck Squamous Cell Carcinoma Through miR-642b-3p.Neoplasia. 2019 Feb;21(2):216-229. doi: 10.1016/j.neo.2018.12.001. Epub 2019 Jan 7. Neoplasia. 2019. PMID: 30622052 Free PMC article.
-
Targeting Immune-Mediated Dormancy: A Promising Treatment of Cancer.Front Oncol. 2019 Jun 26;9:498. doi: 10.3389/fonc.2019.00498. eCollection 2019. Front Oncol. 2019. PMID: 31297335 Free PMC article. Review.
-
[The role of cellular plasticity as the crucial motor for the metastasis of differentiated carcinomas].Postepy Biochem. 2021 Apr 28;67(2):95-103. doi: 10.18388/pb.2021_378. Print 2021 Jun 30. Postepy Biochem. 2021. PMID: 34378893 Review. Polish.
-
Activation of anaphase-promoting complex by p53 induces a state of dormancy in cancer cells against chemotherapeutic stress.Oncotarget. 2016 May 3;7(18):25478-92. doi: 10.18632/oncotarget.8172. Oncotarget. 2016. PMID: 27009858 Free PMC article.
-
Tumor dormancy: EMT beyond invasion and metastasis.Genesis. 2024 Feb;62(1):e23552. doi: 10.1002/dvg.23552. Epub 2023 Sep 30. Genesis. 2024. PMID: 37776086 Review.
Cited by
-
Glucocorticoid receptor: a harmonizer of cellular plasticity in breast cancer-directs the road towards therapy resistance, metastatic progression and recurrence.Cancer Metastasis Rev. 2024 Mar;43(1):481-499. doi: 10.1007/s10555-023-10163-6. Epub 2024 Jan 3. Cancer Metastasis Rev. 2024. PMID: 38170347 Review.
-
Defined Mathematical Relationships Among Cancer Cells Suggest Modular Growth in Tumor Progression and Highlight Developmental Features Consistent With a Para-Embryonic Nature of Cancer.Front Cell Dev Biol. 2020 Aug 28;8:804. doi: 10.3389/fcell.2020.00804. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32984319 Free PMC article.
-
The Post-Translational Regulation of Epithelial-Mesenchymal Transition-Inducing Transcription Factors in Cancer Metastasis.Int J Mol Sci. 2021 Mar 30;22(7):3591. doi: 10.3390/ijms22073591. Int J Mol Sci. 2021. PMID: 33808323 Free PMC article. Review.
-
C4orf47 contributes to the dormancy of pancreatic cancer under hypoxic conditions.J Cancer. 2023 Jan 9;14(2):306-317. doi: 10.7150/jca.78993. eCollection 2023. J Cancer. 2023. PMID: 36741255 Free PMC article.
-
Inflammation-Driven Breast Tumor Cell Plasticity: Stemness/EMT, Therapy Resistance and Dormancy.Front Oncol. 2021 Jan 26;10:614468. doi: 10.3389/fonc.2020.614468. eCollection 2020. Front Oncol. 2021. PMID: 33585241 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

