Ustekinumab-associated disseminated verrucae
- PMID: 30456278
- PMCID: PMC6232639
- DOI: 10.1016/j.jdcr.2018.08.014
Ustekinumab-associated disseminated verrucae
Keywords: APC, antigen presenting cell; HPV, human papilloma virus; IFN, interferon; IL, interleukin; PDT, photodynamic therapy; TNF, tumor necrosis factor; Th, helper T cell; biologics; immunosuppression; psoriasis; ustekinumab; verrucae.
Figures
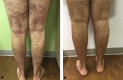

References
-
- Griffiths C.E., Strober B.E., van de Kerkhof P., ACCEPT Study Group Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–128. - PubMed
-
- Galloway J.B., Hyrich K.L., Mercer L.K., BSRBR Control Centre Consortium; British Society for Rheumatology Biologics Register Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 2011;50(1):124–131. - PMC - PubMed
-
- Koc E., Tunca M., Arda E. Multiple widespread warts due to etanercept treatment in a psoriatic patient: a case report. J Turk Acad Dermatol. 2008;2(1):82103c.
-
- Leonardi C.L., Kimball A.B., Papp K.A. Efficacy and safety of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. - PubMed
-
- Papp K.A., Griffiths C.E., Gordon K., PHOENIX 1 Investigators; PHOENIX 2 Investigators; ACCEPT Investigators Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168(4):844–854. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
