21-Gene assay as predictor of chemotherapy benefit in HER2-negative breast cancer
- PMID: 30456299
- PMCID: PMC6235896
- DOI: 10.1038/s41523-018-0090-6
21-Gene assay as predictor of chemotherapy benefit in HER2-negative breast cancer
Abstract
The NSABP B-20 prospective-retrospective study of the 21-gene Oncotype DX Breast Cancer Recurrence Score® test predicted benefit from addition of chemotherapy to tamoxifen in node-negative, estrogen-receptor positive breast cancer when recurrence score (RS) was ≥31. HER2 is a component of the RS algorithm with a positive coefficient and contributes to higher RS values. Accrual to B-20 occurred prior to routine testing for HER2, so questions have arisen regarding assay performance if HER2-positive patients were identified and excluded. We report an exploratory reanalysis of the B-20, 21-gene study following exclusion of such patients. Patients were considered HER2 positive if quantitative RT-PCR for HER2 was ≥11.5 units, and excluded from re-analyses performed using the original cutoffs: <18, 18-30, ≥31, and the TAILORx cutoffs: <11, 11-25, >25. The endpoint remained distant recurrence-free interval (DRFI) as in the original study. Distribution was estimated via the Kaplan-Meier method and compared via log-rank test. Multivariate Cox proportional hazards models estimated chemotherapy benefit in each group. In the RS < 18 and 18-30 groups, 1.7 and 6.7% were HER2 positive. In the RS ≥ 31 group, 41% were HER2 positive. Exclusion resulted in fewer events, with loss of significance for benefit from chemotherapy in the overall HER2-negative cohort (log-rank P = 0.06), but substantial benefit from chemotherapy remained in the RS ≥ 31 cohort (HR = 0.18; 95% CI: 0.07-0.47) and the RS > 25 cohort (HR = 0.28; 95% CI: 0.12-0.64). No benefit from chemotherapy was evident in the other RS groups. Following exclusion of HER2-positive patients based on RT-PCR expression, substantial benefit of chemotherapy remained for RS ≥ 31 as originally employed, and with RS > 25 employed in TAILORx.
Conflict of interest statement
Dr. Mamounas: Genomic Health, Inc.: Consultant, Honoraria (Speaker); Biotheranostics: Consultant. Dr. Paik discloses being listed as one of the inventors of OncotypeDx in the patents, but all rights have been transferred to NSABP Foundation, resulting in no financial interest. Dr. Shak: Genomic Health, Inc.: Full-time employee and shareholder. Dr. Crager: Employment at Genomic Health, Inc., and ownership of company stock. Dr. Baehner: Employee and minor shareholder of Genomic Health, Inc. All other authors declare no other potential competing interest(s).
Figures
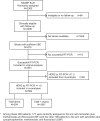

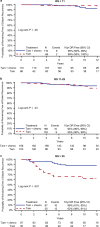
References
-
- Albain KS, et al. Prognostic and 286 predictive value of the 21-gene recurrence score assay in postmenopausal women with node-287 positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis 288 of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

