Cardiac Progenitor Cell-Derived Extracellular Vesicles Reduce Infarct Size and Associate with Increased Cardiovascular Cell Proliferation
- PMID: 30456736
- PMCID: PMC6394631
- DOI: 10.1007/s12265-018-9842-9
Cardiac Progenitor Cell-Derived Extracellular Vesicles Reduce Infarct Size and Associate with Increased Cardiovascular Cell Proliferation
Abstract
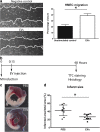
Cell transplantation studies have shown that injection of progenitor cells can improve cardiac function after myocardial infarction (MI). Transplantation of human cardiac progenitor cells (hCPCs) results in an increased ejection fraction, but survival and integration are low. Therefore, paracrine factors including extracellular vesicles (EVs) are likely to contribute to the beneficial effects. We investigated the contribution of EVs by transplanting hCPCs with reduced EV secretion. Interestingly, these hCPCs were unable to reduce infarct size post-MI. Moreover, injection of hCPC-EVs did significantly reduce infarct size. Analysis of EV uptake showed cardiomyocytes and endothelial cells primarily positive and a higher Ki67 expression in these cell types. Yes-associated protein (YAP), a proliferation marker associated with Ki67, was also increased in the entire infarcted area. In summary, our data suggest that EV secretion is the driving force behind the short-term beneficial effect of hCPC transplantation on cardiac recovery after MI.
Keywords: Angiogenesis; Cardiac progenitor cells; Cardiomyocytes; Endoglin; Extracellular vesicles; Myocardial infarction; Proliferation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Comment in
-
Cardiac Progenitor Cell-Derived Extracellular Vesicles: a Rising Star for Cardiac Repair and Regeneration.J Cardiovasc Transl Res. 2019 Feb;12(1):3-4. doi: 10.1007/s12265-018-9862-5. Epub 2019 Jan 4. J Cardiovasc Transl Res. 2019. PMID: 30610671 No abstract available.
References
-
- (2018). WHO | Global Health Observatory (GHO) data. WHO.
-
- den Haan MC, Grauss RW, Smits AM, et al. Cardiomyogenic differentiation-independent improvement of cardiac function by human cardiomyocyte progenitor cell injection in ischaemic mouse hearts. Journal of Cellular and Molecular Medicine. 2012;16:1508–1521. doi: 10.1111/j.1582-4934.2011.01468.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

