Glycaemic efficacy and safety of linagliptin compared to a basal-bolus insulin regimen in patients with type 2 diabetes undergoing non-cardiac surgery: A multicentre randomized clinical trial
- PMID: 30456796
- PMCID: PMC7231260
- DOI: 10.1111/dom.13587
Glycaemic efficacy and safety of linagliptin compared to a basal-bolus insulin regimen in patients with type 2 diabetes undergoing non-cardiac surgery: A multicentre randomized clinical trial
Abstract
Aims: The use of incretin-based therapy, rather than or complementary to, insulin therapy is an active area of research in hospitalized patients with type 2 diabetes (T2D). We determined the glycaemic efficacy and safety of linagliptin compared to a basal-bolus insulin regimen in hospitalized surgical patients with T2D.
Materials and methods: This prospective open-label multicentre study randomized T2D patients undergoing non-cardiac surgery with admission blood glucose (BG) of 7.8 to 22.2 mmol/L who were under treatment with diet, oral agents or total insulin dose (TDD) ≤ 0.5 units/kg/day to either linagliptin (n = 128) daily or basal-bolus (n = 122) with glargine once daily and rapid-acting insulin before meals. Both groups received supplemental insulin for BG > 7.8 mmol/L. The primary endpoint was difference in mean daily BG between groups.
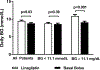
Results: Mean daily BG was higher in the linagliptin group compared to the basal-bolus group (9.5 ± 2.6 vs 8.8 ± 2.3 mmol/L/dL, P = 0.03) with a mean daily BG difference of 0.6 mmol/L (95% confidence interval 0.04, 1.2). In patients with randomization BG < 11.1 mmol/L (63% of cohort), mean daily BG was similar in the linagliptin and basal-bolus groups (8.9 ± 2.3 vs 8.7 ± 2.3 mmol/L, P = 0.43); however, patients with BG ≥ 11.1 mmol/L who were treated with linagliptin had higher BG compared to the basal-bolus group (10.9 ± 2.6 vs 9.2 ± 2.2 mmol/L, P < 0.001). Linagliptin resulted in fewer hypoglycaemic events (1.6% vs 11%, P = 0.001; 86% relative risk reduction), with similar supplemental insulin and fewer daily insulin injections (2.0 ± 3.3 vs 3.1 ± 3.3, P < 0.001) compared to the basal-bolus group.
Conclusions: For patients with T2D undergoing non-cardiac surgery who presented with mild to moderate hyperglycaemia (BG < 11.1 mmol/L), daily linagliptin is a safe and effective alternative to multi-dose insulin therapy, resulting in similar glucose control with lower hypoglycaemia.
Keywords: DPP-IV inhibitor; glycaemic control; incretin therapy; randomised trial.
© 2018 John Wiley & Sons Ltd.
Figures

References
-
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982. - PubMed
-
- Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. Jama. 2003;290(15):2041–2047. - PubMed
-
- Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31(2):359–366. - PubMed
-
- Pomposelli JJ, Baxter JK 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22(2):77–81. - PubMed
-
- Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26(1):57–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

