Synergistic Antitumor Effect on Bladder Cancer by Rational Combination of Programmed Cell Death 1 Blockade and CRISPR-Cas9-Mediated Long Non-Coding RNA Urothelial Carcinoma Associated 1 Knockout
- PMID: 30457360
- PMCID: PMC12199628
- DOI: 10.1089/hum.2018.048
Synergistic Antitumor Effect on Bladder Cancer by Rational Combination of Programmed Cell Death 1 Blockade and CRISPR-Cas9-Mediated Long Non-Coding RNA Urothelial Carcinoma Associated 1 Knockout
Abstract
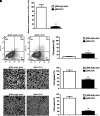
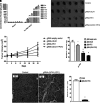
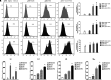
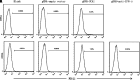
Targeted therapy produces objective responses in bladder cancer patients, although the responses can be short. Meanwhile, response rates to immune therapy are lower, but the effects are more durable. Based on these findings, it was hypothesized that urothelial carcinoma associated 1 (UCA1)-targeted therapy could synergize with programmed cell death 1 (PD-1) blockade to enhance antitumor activity. To test this hypothesis, the effects of CRISPR-Cas9 targeting of UCA1 and PD-1 were assessed in vitro and in vivo. It was found that gRNA/cas9-targeted UCA1 induced apoptosis of 5637 bladder cancer cells, whereas PD-1 gene knockout could be achieved by electroporation of gRNA/cas9 targeting PD-1, as detected by polymerase chain reaction. In 5637 cell-xenografted humanized SCID mice, stimulation with CRISPR-Cas9 systems, immune phenotypes, and cytokine expression of human dendritic cells (DCs) was detected by flow cytometry, and polymerase chain reaction, respectively. The results of these assays suggested that the gRNA/cas9 treatment upregulated expression of CD80, CD83, and CD86 and significantly increased interleukin (IL)-6, IL-12, and IL-23 and tumor necrosis factor alpha mRNA levels. Co-administration of anti-PD-1 and anti-UCA1 treatment suppressed tumor growth and markedly improved survival of 5637 xenografted mice. Additionally, the combination treatment increased interferon gamma production by T cells that subsequently enhanced the expression of Th1-associated immune-stimulating genes to reduce transcription of regulatory/suppressive immune genes and reshape the tumor microenvironment from an immunosuppressive to a stimulatory state. Finally, anti-UCA1 treatment was shown to induce interferon gamma-dependent programmed cell death ligand 1 expression within 5637 xenograft tumors in vivo. Together, these results demonstrate potent synergistic effects of a combination therapy using LncRNA UCA1-targeted therapy and immune checkpoint blockade of PD-1, thus supporting the translational potential of this combination strategy for clinical treatment of bladder cancer.
Keywords: LncRNA; PD-1; checkpoint blockade; combination therapy; targeted therapy.
Figures




References
-
- Siegel R, Ma J, Zou Z, et al. Cancer statistics. CA Cancer J Clin 2014;64:9–29. - PubMed
-
- Oing C, Rink M, Oechsle K, et al. Second line chemotherapy for advanced and metastatic urothelial carcinoma: vinflunine and beyond—a comprehensive review of the current literature. J Urol 2016;195:254–263. - PubMed
-
- Shah JB, McConkey DJ, Dinney CP. New strategies in muscle-invasive bladder cancer: on the road to personalized medicine. Clin Cancer Res 2011;17:2608–2612. - PubMed
-
- McConkey D, Choi W, Dinney C, et al. New insights into subtypes of invasive bladder cancer: considerations of the clinician. Eur Urol 2014;66:609–610. - PubMed
-
- Carosella ED, Ploussard G, LeMaoult J, et al. A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol 2015;68:267–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

