Novel Type of Chronic Wasting Disease Detected in Moose (Alces alces), Norway
- PMID: 30457526
- PMCID: PMC6256397
- DOI: 10.3201/eid2412.180702
Novel Type of Chronic Wasting Disease Detected in Moose (Alces alces), Norway
Abstract
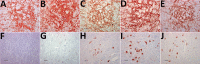
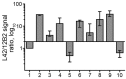
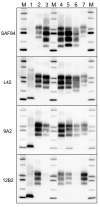
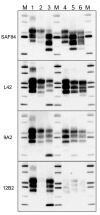
Chronic wasting disease (CWD) persists in cervid populations of North America and in 2016 was detected for the first time in Europe in a wild reindeer in Norway. We report the detection of CWD in 3 moose (Alces alces) in Norway, identified through a large scale surveillance program. The cases occurred in 13-14-year-old female moose, and we detected an abnormal form of prion protein (PrPSc) in the brain but not in lymphoid tissues. Immunohistochemistry revealed that the moose shared the same neuropathologic phenotype, characterized by mostly intraneuronal deposition of PrPSc. This pattern differed from that observed in reindeer and has not been previously reported in CWD-infected cervids. Moreover, Western blot revealed a PrPSc type distinguishable from previous CWD cases and from known ruminant prion diseases in Europe, with the possible exception of sheep CH1641. These findings suggest that these cases in moose represent a novel type of CWD.
Keywords: Alces alces; CWD; Nor16CWD; Norway; TSE; cervid; chronic wasting disease; moose; prions; reindeer; transmissible spongiform encephalopathy; zoonoses.
Figures


References
-
- EFSA Panel on Biological Hazards. Scientific opinion on chronic wasting disease (CWD) in cervids. EFSA J. 2017;15:4667.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

