Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant
- PMID: 30457530
- PMCID: PMC6256372
- DOI: 10.3201/eid2412.180937
Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant
Abstract
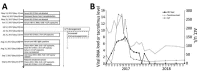
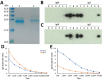
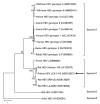
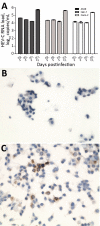
All hepatitis E virus (HEV) variants reported to infect humans belong to the species Orthohepevirus A (HEV-A). The zoonotic potential of the species Orthohepevirus C (HEV-C), which circulates in rats and is highly divergent from HEV-A, is unknown. We report a liver transplant recipient with hepatitis caused by HEV-C infection. We detected HEV-C RNA in multiple clinical samples and HEV-C antigen in the liver. The complete genome of the HEV-C isolate had 93.7% nt similarity to an HEV-C strain from Vietnam. The patient had preexisting HEV antibodies, which were not protective against HEV-C infection. Ribavirin was an effective treatment, resulting in resolution of hepatitis and clearance of HEV-C viremia. Testing for this zoonotic virus should be performed for immunocompromised and immunocompetent patients with unexplained hepatitis because routine hepatitis E diagnostic tests may miss HEV-C infection. HEV-C is also a potential threat to the blood product supply.
Keywords: HEV; Hong Kong; chronic hepatitis; hepatitis; hepatitis E; immunocompromised; liver transplant; rat hepatitis E virus; rodents; viruses; zoonoses.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

