Comprehensive Glycoproteomic Analysis of Chinese Hamster Ovary Cells
- PMID: 30457839
- PMCID: PMC6440468
- DOI: 10.1021/acs.analchem.8b03520
Comprehensive Glycoproteomic Analysis of Chinese Hamster Ovary Cells
Abstract
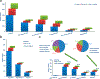
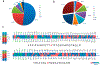
The Chinese hamster ovary (CHO) cell line is a major expression system for the production of therapeutic proteins, the majority of which are glycoproteins, such as antibodies and erythropoietin (EPO). The characterization glycosylation profile of therapeutic proteins produced from engineered CHO cells and therapeutic functions, as well as side effects, are critical to understand the important roles of glycosylation. In this study, a large scale glycoproteomic workflow was established and applied to CHO-K1 cells expressing EPO. The workflow includes enrichment of intact glycopeptides from CHO-K1 cell lysate and medium using hydrophilic enrichment, fractionation of the obtained intact glycopeptides (IGPs) by basic reversed phase liquid chromatography (bRPLC), analyzing the glycopeptides using LC-MS/MS, and annotating the results by GPQuest 2.0. A total of 10 338 N-linked glycosite-containing IGPs were identified, representing 1162 unique glycosites in 530 glycoproteins, including 71 unique atypical N-linked IGPs on 18 atypical N-glycosylation sequons with an overrepresentation of the N-X-C motifs. Moreover, we compared the glycoproteins from CHO cell lysate with those from medium using the in-depth N-linked glycoproteome data. The obtained large scale glycoproteomic data from intact N-linked glycopeptides in this study is complementary to the genomic, proteomic, and N-linked glycomic data previously reported for CHO cells. Our method has the potential to monitor the production of recombinant therapeutic glycoproteins.
Figures



Similar articles
-
The GalNAc-type O-Glycoproteome of CHO cells characterized by the SimpleCell strategy.Mol Cell Proteomics. 2014 Dec;13(12):3224-35. doi: 10.1074/mcp.M114.041541. Epub 2014 Aug 4. Mol Cell Proteomics. 2014. PMID: 25092905 Free PMC article.
-
Glycoproteomic Characterization of FUT8 Knock-Out CHO Cells Reveals Roles of FUT8 in the Glycosylation.Front Chem. 2021 Oct 29;9:755238. doi: 10.3389/fchem.2021.755238. eCollection 2021. Front Chem. 2021. PMID: 34778211 Free PMC article.
-
Comprehensive N- and O-Glycoproteomic Analysis of Multiple Chinese Hamster Ovary Host Cell Lines.J Proteome Res. 2022 Oct 7;21(10):2341-2355. doi: 10.1021/acs.jproteome.2c00207. Epub 2022 Sep 21. J Proteome Res. 2022. PMID: 36129246
-
[Recent advances in glycopeptide enrichment and mass spectrometry data interpretation approaches for glycoproteomics analyses].Se Pu. 2021 Oct;39(10):1045-1054. doi: 10.3724/SP.J.1123.2021.06011. Se Pu. 2021. PMID: 34505426 Free PMC article. Review. Chinese.
-
Glycoengineering Chinese hamster ovary cells: a short history.Biochem Soc Trans. 2021 Apr 30;49(2):915-931. doi: 10.1042/BST20200840. Biochem Soc Trans. 2021. PMID: 33704400 Free PMC article. Review.
Cited by
-
Databases and Bioinformatic Tools for Glycobiology and Glycoproteomics.Int J Mol Sci. 2020 Sep 14;21(18):6727. doi: 10.3390/ijms21186727. Int J Mol Sci. 2020. PMID: 32937895 Free PMC article. Review.
-
Towards structure-focused glycoproteomics.Biochem Soc Trans. 2021 Feb 26;49(1):161-186. doi: 10.1042/BST20200222. Biochem Soc Trans. 2021. PMID: 33439247 Free PMC article. Review.
-
An Integrated Workflow for Global, Glyco-, and Phospho-proteomic Analysis of Tumor Tissues.Anal Chem. 2020 Jan 21;92(2):1842-1849. doi: 10.1021/acs.analchem.9b03753. Epub 2020 Jan 3. Anal Chem. 2020. PMID: 31859488 Free PMC article.
-
Diverse perspectives on proteomic posttranslational modifications to address EGFR-TKI resistance in non-small cell lung cancer.Front Cell Dev Biol. 2024 Dec 24;12:1436033. doi: 10.3389/fcell.2024.1436033. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39777265 Free PMC article. Review.
-
Characterization of the Ubiquitin-Modified Proteome of Recombinant Chinese Hamster Ovary Cells in Response to Endoplasmic Reticulum Stress.Biotechnol J. 2024 Nov;19(11):e202400413. doi: 10.1002/biot.202400413. Biotechnol J. 2024. PMID: 39623727 Free PMC article.
References
-
- Walsh G Nat. Biotechnol. 2014, 32, 992. - PubMed
-
- Chung CY; Yin B; Wang Q; Chuang KY; Chu JH; Betenbaugh MJ Biochem. Biophys. Res. Commun. 2015, 463, 211–215. - PubMed
-
- Lewis NE; Liu X; Li Y; Nagarajan H; Yerganian G; O’Brien E; Bordbar A; Roth AM; Rosenbloom J; Bian C; Xie M; Chen W; Li N; Baycin-Hizal D; Latif H; Forster J; Betenbaugh MJ; Famili I; Xu X; Wang J; Palsson BO Nat. Biotechnol. 2013, 31, 759–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

