A pilot trial of RNS60 in amyotrophic lateral sclerosis
- PMID: 30458059
- PMCID: PMC6379136
- DOI: 10.1002/mus.26385
A pilot trial of RNS60 in amyotrophic lateral sclerosis
Abstract
Introduction: RNS60 is a novel immune-modulatory agent that has shown neuroprotective effects in amytrophic lateral sclerosis (ALS) preclinical models. RNS60 is administered by weekly intravenous infusion and daily nebulization. The objective of this pilot open-label trial was to test the feasibility, safety, and tolerability of long-term RNS60 administration in ALS patients.
Methods: The planned treatment duration was 23 weeks and the primary outcomes were safety and tolerability. Secondary outcomes included PBR28 positron emission tomography (PET) imaging and plasma biomarkers of inflammation.
Results: Sixteen participants with ALS received RNS60 and 13 (81%) completed 23 weeks of RNS60 treatment. There were no serious adverse events and no participants withdrew from the trial due to drug-related adverse events. There were no significant changes in the biomarkers.
Discussion: Long-term RNS60 administration was safe and well-tolerated. A large, multicenter, phase II trial of RNS60 is currently enrolling participants to test the effects of RNS60 on ALS biomarkers and disease progression. Muscle Nerve 59:303-308, 2019.
Keywords: ALS; PBR28; clinical trial; motor neuron disease (MND); neuroinflammation.
© 2018 Wiley Periodicals, Inc.
Figures

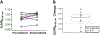

References
-
- Hardiman O, Al-Chalabi A, Brayne C, Beghi E, van den Berg LH, Chio A, et al. The changing picture of amyotrophic lateral sclerosis: lessons from European registers. Journal of neurology, neurosurgery, and psychiatry. 2017;88(7):557–563. - PubMed
-
- Writing G, Edaravone ALSSG. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505–512. - PubMed
-
- Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57(7):1282–1289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

